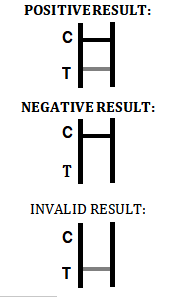China iGFBP-1 Rapid Test Device/Strip (Vaginal Secretion)
Product Main Parameters
| Product Name | iGFBP-1 Rapid Test Device |
|---|---|
| Used for | In vitro diagnostic use |
| Specimen | Vaginal secretion |
| Packing | 20 tests/box, 1 test/polybag |
| MOQ | 1000 tests |
Common Product Specifications
| Storage | 2‐30°C |
|---|---|
| Stability | Do not freeze |
| Interpretation | Positive, Negative, Invalid |
Product Manufacturing Process
The manufacturing process of the China iGFBP-1 Rapid Test Device/Strip involves immunochromatographic techniques to ensure high specificity and sensitivity. The test utilizes monoclonal antibodies specific for iGFBP-1. After precise formulation and quality control checks, test kits are assembled, sealed, and stored under recommended conditions to maintain stability. Advanced studies in chromatography and immunoassay methodologies ensure that each strip meets rigorous manufacturing standards, providing reliable diagnostic results crucial in medical diagnostics.
Product Application Scenarios
The China iGFBP-1 Rapid Test Device is essential in obstetric care for detecting rupture of fetal membranes (ROM), a critical factor in managing preterm birth risks. Employed by healthcare professionals, this test aids decision-making regarding the timing of delivery and potential interventions. Studies indicate that accurate detection of iGFBP-1 in vaginal secretions significantly improves diagnostic accuracy, enhancing maternal-fetal outcomes. Its role in identifying ROM timely can reduce complications, promoting better healthcare practices in obstetrics across diverse medical settings.
Product After-Sales Service
We offer comprehensive after-sales service, including technical support and user guidance. Our team is available to ensure customer satisfaction and solve any queries regarding the China iGFBP-1 Rapid Test Device. Contact us for any issues, and expect prompt service.
Product Transportation
Products are shipped with care to maintain optimal conditions. They are transported in temperature-controlled environments to prevent exposure to extreme temperatures, ensuring product integrity upon arrival.
Product Advantages
- High specificity and sensitivity.
- Rapid and easy-to-use.
- Optimal for professional in vitro diagnostic settings.
- Reliable detection of iGFBP-1 in vaginal secretions.
Product FAQ
- What is the primary use of the China iGFBP-1 Rapid Test? The test is primarily used to detect the presence of iGFBP-1 in vaginal secretions, helping diagnose the rupture of fetal membranes in pregnant women.
- How should the test kits be stored? Store the kits at temperatures between 2‐30°C, ensuring they remain sealed until use. Avoid freezing to maintain test efficacy.
- Is the test qualitative or quantitative? The China iGFBP-1 Rapid Test is qualitative, meaning it indicates the presence or absence of iGFBP-1, not the concentration levels.
- Who can perform this test? This test is intended for use by healthcare professionals trained in handling in vitro diagnostic tests.
- Can I use the test kit after the expiration date? Using the test kit after its expiration date is not recommended as it may lead to inaccurate results.
- Why is iGFBP-1 detection important during pregnancy? Detecting iGFBP-1 helps diagnose fetal membrane rupture, which can lead to preterm birth if not managed properly.
- What should I do if the test shows an invalid result? Repeat the test with a new device. If issues persist, contact your distributor for assistance.
- What factors can affect test results? Insufficient specimen volume, improper procedure, or expired tests can impact results. Follow guidelines carefully.
- How long does it take to read results? Results should be read within the specified time on the instructions to ensure accuracy.
- Can this test be used for non-pregnant patients? The China iGFBP-1 Rapid Test is specifically designed for pregnant women to detect fetal membrane rupture.
Product Hot Topics
- The Role of China iGFBP in Obstetric Diagnostics: The China iGFBP-1 Rapid Test Device is pivotal for healthcare providers in obstetric diagnostics. By detecting iGFBP-1 in vaginal secretions, it aids in identifying the rupture of fetal membranes (ROM), a critical factor in preventing preterm births. The test's reliable results contribute to more informed clinical decisions, significantly impacting maternal and neonatal health outcomes.
- Enhancing Prenatal Care with China iGFBP Testing: Integrating the China iGFBP-1 Rapid Test into prenatal care protocols provides healthcare professionals with a powerful diagnostic tool. Detecting fetal membrane rupture early enables timely interventions, reducing risks associated with preterm labor. The test's accessibility and ease of use make it an essential component of comprehensive prenatal care.
- China iGFBP-1: A Game-Changer in Fetal Membrane Health Monitoring: The China iGFBP-1 Rapid Test's introduction represents a significant advancement in monitoring fetal membrane health. Its ability to promptly and accurately detect iGFBP-1 levels helps physicians manage pregnancies with complications effectively, ensuring better health outcomes for mothers and babies.
- Understanding the Science Behind China iGFBP Testing: The science behind the China iGFBP-1 Rapid Test involves sophisticated immunochromatographic techniques, providing reliable detection of iGFBP-1 indicators. This technological innovation supports physicians in diagnosing fetal membrane issues efficiently, contributing to higher standards of obstetric care.
- Future Directions in Obstetric Diagnostics with China iGFBP: As obstetric care evolves, the China iGFBP-1 Rapid Test is set to play an integral role in refining diagnostic approaches. Its ongoing development promises enhanced accuracy and expanded applications, paving the way for future advancements in maternal-fetal medicine.
Image Description