China Influenza Test: SARS-COV-2/Influenza AB Combo
Product Main Parameters
| Brand | QL |
|---|---|
| Certificate | CE |
| Specimen | Nasopharyngeal swabs/Nasal swab |
| Pack | 20T |
| Reading Time | 10 minutes |
| Contents | Cassette, Buffer, Package insert |
| Storage | 2-30°C |
| Shelf Life | 2 years |
Common Product Specifications
| Test Format | Rapid visual immunoassay |
|---|---|
| Detection | Influenza A, B, and SARS-COV-2 Antigens |
| Control Band | Included |
Product Manufacturing Process
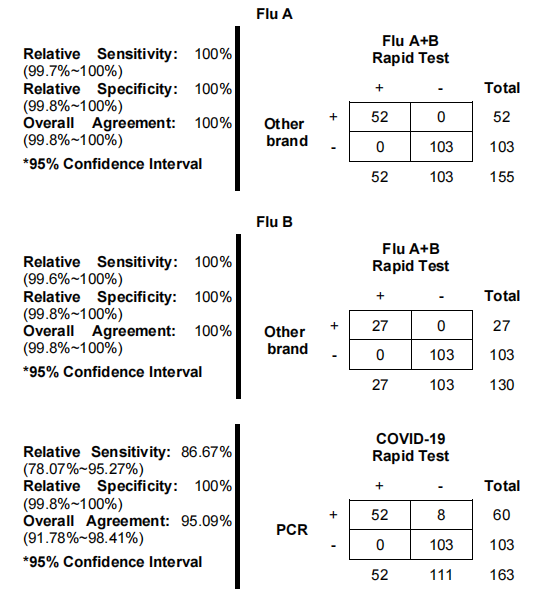
Based on current authoritative research, the production of rapid influenza tests involves multiple rigorous stages, including antigen extraction, antibody development, and the assembly of test components into devices. Thorough quality control measures ensure test sensitivity and specificity. The process also includes stability testing and environmental assessments to ensure durability across different climatic conditions.
Product Application Scenarios
Clinical usage of the China Influenza Test is primarily in hospitals, clinics, and at home healthcare settings for rapid diagnosis of influenza and COVID-19. Its rapid results facilitate timely medical decision-making, especially in peak flu seasons or outbreaks. Effective in managing public health by enabling quick identification of viral infections, guiding isolation, and treatment measures.
Product After-sales Service
QL Biotech offers comprehensive after-sales support, including customer service for technical inquiries, product replacements for defects, and additional user training. Contact avenues include email and phone support with a dedicated team ready to assist globally.
Product Transportation
The product is transported under controlled conditions to maintain optimal shelf life and efficacy. Packaging ensures protection against environmental factors during transit, and expedited shipping options are available.
Product Advantages
- Rapid results within 10 minutes for timely decision-making.
- High specificity and sensitivity conforming to CE standards.
- Convenient nasopharyngeal or nasal swab specimen collection.
- Dual detection of Influenza A, B, and COVID-19 antigens.
Product FAQ
- What types of specimens are required? The China Influenza Test requires nasopharyngeal or nasal swabs for accurate testing.
- How quickly are results available? Results are available in just 10 minutes, allowing for expedited clinical decision-making.
- Is the test CE certified? Yes, the test is CE certified, ensuring adherence to European quality standards.
- What is the shelf life of the test kit? The shelf life is 2 years when stored under recommended conditions (2-30°C).
- How should the test kit be stored? Store the kit in a temperature range from 2-30°C to maintain efficacy.
- Can the test detect both Influenza A and B? Yes, it detects both Influenza A and B, along with SARS-COV-2.
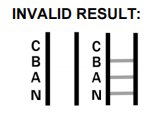
- What should be done in case of an invalid result? An invalid result indicates a test failure. Repeat with a new test, ensuring correct procedure.
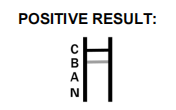
- How are positive results indicated? Positive results for specific viruses are indicated by colored bands appearing in designated regions on the test device.
- Is further testing needed after a negative result? If clinical suspicion remains, additional testing may be necessary, as rapid tests may occasionally yield false negatives.
- Who can perform the test? The test is designed for use by healthcare professionals familiar with the procedure and interpretation of rapid diagnostic tests.
Product Hot Topics
- Discussion on Test Accuracy: With increasing global demand for rapid diagnostic solutions, the accuracy of the China Influenza Test is a critical topic. It offers reliable results through CE certified processes, ensuring comprehensive detection of viral antigens for both influenza types and SARS-COV-2. This test stands as a viable option for healthcare facilities needing swift diagnostic solutions.
- Global Distribution Challenges: The logistics of distributing the China Influenza Test worldwide raise opportunities and challenges. Ensuring efficient transportation while maintaining the integrity of the product is vital. Exploring partnerships with international distributors, QL Biotech aims to streamline delivery processes, enabling rapid access to essential diagnostic tools amid global health challenges.
- Economic Impact of Rapid Tests: Rapid influenza tests such as those from China are reshaping healthcare economics by offering cost-effective, high-speed diagnostics. This leads to reduced hospital stays and optimized resource allocation, suggesting a shift towards broader adoption in global healthcare systems.
- Advancements in Test Technology: Ongoing advancements in rapid testing technology, focusing on enhancing sensitivity and specificity, position the China Influenza Test at the forefront. Innovations continue to reduce process time while expanding detection capabilities, catering to emerging viral threats.
- Adapting to Viral Mutations: With viral mutations a continuous concern, the adaptability of the China Influenza Test is essential. Through constant R&D, QL Biotech ensures the test's components effectively adapt to genetic variations, preserving its effectiveness across evolving viral strains.
- Patients' Experience with Testing: User-friendly design and ease of use are pivotal for patient compliance with the China Influenza Test. Feedback indicates satisfactory specimen collection experiences, highlighting its practical design for both professionals and patients in various healthcare settings.
- Regulatory Standards and Compliance: Compliance with international standards like CE certification reassures users of the China Influenza Test's quality. Regulatory adherence guarantees its acceptance and reliability in diverse healthcare markets globally.
- Training for Effective Use: QL Biotech's investment in training resources ensures that healthcare professionals are adequately equipped to utilize the China Influenza Test efficiently. This commitment ranges from instructional materials to hands-on workshops, enhancing user competence.
- Combatting Seasonal Outbreaks: In regions prone to seasonal influenza outbreaks, deploying rapid diagnostic tools like the China Influenza Test is crucial. Its swift results enable proactive measures, curtailing infection spread and assisting in effective public health management.
- Community Health Impacts: The wide availability of the China Influenza Test contributes significantly to community health surveillance, enhancing the ability to track and respond to influenza trends. This positions it as an integral component in public health initiatives globally.
Image Description