China OEM Fecal H. Pylori AG Test Device
Product Main Parameters
| Parameter | Description |
|---|---|
| Principle | Immunochromatography |
| Specimen Type | Fecal Samples |
| Result Time | 15-20 minutes |
| Sensitivity | High |
| Specificity | High |
Common Product Specifications
| Component | Included Items |
|---|---|
| Test Kit | Test Cassette, Buffer Solution, Dropper |
| Package Size | 25 Tests |
| Storage Temperature | 2-30°C |
| Reading Time | 15 minutes |
Product Manufacturing Process
The manufacturing process of the China OEM Fecal H. Pylori AG Test Device involves sophisticated immunochromatographic techniques. Initially, high-affinity antibodies are developed and immobilized on the test cassette membrane. These antibodies are designed to interact specifically with H. pylori antigens. The production process ensures high sensitivity and specificity by rigorously testing each batch of antibodies. The device is assembled under controlled conditions, with quality checks at every stage to ensure consistency and reliability. The final product undergoes validation using various samples to confirm its efficacy in clinical scenarios, ensuring that it delivers accurate results within 15-20 minutes of testing.
Product Application Scenarios
The China OEM Fecal H. Pylori AG Test Device is widely used in clinical settings for the detection of Helicobacter pylori infections. It is particularly beneficial in gastroenterology clinics and hospitals, where non-invasive tests are preferred for patient comfort. The device is suitable for initial screenings and for confirming the eradication of H. pylori post-treatment. Its portability allows it to be used in remote or resource-limited settings where traditional diagnostic methods are not feasible. It is an essential tool in areas with high prevalence of H. pylori-related diseases, facilitating timely diagnosis and management of these conditions.
Product After-sales Service
QL Biotech provides comprehensive after-sales support for the China OEM Fecal H. Pylori AG Test Device. This includes technical assistance, product training for healthcare professionals, and a responsive customer service team available for troubleshooting and queries. Replacement parts and additional test kits can be ordered directly from QL Biotech, ensuring uninterrupted service and availability.
Product Transportation
All test kits are carefully packaged to preserve their integrity during transport. They are shipped under regulated temperature conditions to prevent any compromise in performance. International shipping is available, and orders are typically dispatched within 3-5 business days.
Product Advantages
- Non-invasive and patient-friendly.
- Rapid results within 15-20 minutes.
- High sensitivity and specificity to minimize false results.
- Cost-effective with no need for specialized equipment.
Product FAQ
- How does the China OEM Fecal H. Pylori AG Test Device work?
It utilizes immunochromatography to detect H. pylori antigens in fecal samples, providing rapid results based on antigen-antibody interactions.
- What is the accuracy of this test device?
The device boasts high sensitivity and specificity, making it reliable for detecting H. pylori infections, though accuracy can be influenced by sample quality and testing adherence.
- Are there any medications that can affect the test results?
Yes, recent intake of antibiotics or proton pump inhibitors can lead to false-negative results. Patients should consult healthcare providers about medication cessation prior to testing.
- Is it suitable for all age groups?
Yes, the test is appropriate for patients of all ages due to its non-invasive nature, though instructions should be followed carefully for accurate results.
- What are the storage requirements for the test kits?
The test kits should be stored at temperatures between 2-30°C, away from direct sunlight and humidity to maintain their efficacy.
- Can the test be used as a confirmatory test?
While useful for preliminary screening, confirmatory tests or further evaluation may be necessary for definitive diagnosis, especially in clinical settings.
- How should I prepare a sample for testing?
A fresh fecal sample should be collected and mixed with the provided buffer solution before applying it to the test cassette.
- Is the test reliable in all environments?
Yes, the robustness of the test allows it to function effectively in diverse environments, although extreme conditions should be avoided during testing.
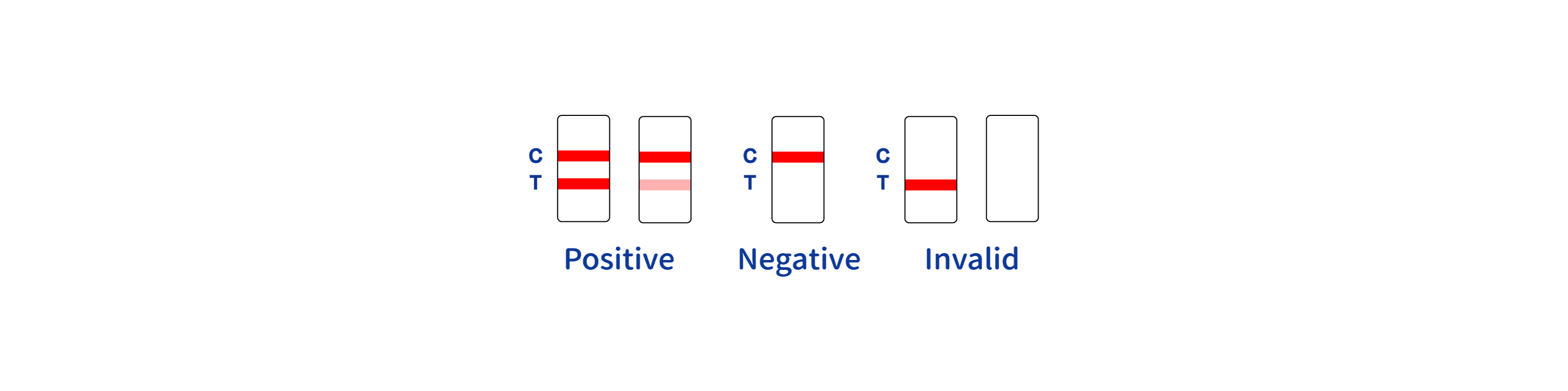
- What if I notice no lines on the test cassette?
This could indicate an error in the procedure. Check the sample volume or technique, and consider retesting with a new device if necessary.
- How do I dispose of the used test kits?
Ensure proper disposal according to local regulations for medical waste to prevent contamination and environmental harm.
Product Hot Topics
- Enhancing Diagnostic Methods in China Through Innovation
China's OEM Fecal H. Pylori AG Test Device represents a significant step forward in medical diagnostics. By providing a non-invasive and highly reliable method of detecting H. pylori, it addresses both patient comfort and diagnostic accuracy. Such technological innovations are crucial in improving healthcare outcomes and streamlining processes in healthcare systems across the globe. By focusing on user-friendly and cost-effective solutions, this test device contributes to China's growing influence in the IVD industry.
- Understanding the Role of H. Pylori in Gastrointestinal Diseases
The prevalence of Helicobacter pylori in gastrointestinal disorders underscores the necessity for accurate diagnostic tools such as the China OEM Fecal H. Pylori AG Test Device. H. pylori, a major cause of peptic ulcers and gastric cancer, requires prompt and precise detection to facilitate effective treatment. Devices like these allow for early intervention, preventing complications and ensuring better patient outcomes.
- The Economic Impact of Rapid Diagnostic Tests in Healthcare
Rapid diagnostic tests like the China OEM Fecal H. Pylori AG Test Device offer both cost savings and efficiency improvements. By reducing the need for invasive procedures, the healthcare system can allocate resources more effectively, resulting in broader access to medical services for patients. These economic benefits make it an attractive option for healthcare providers looking to optimize their operations without compromising on care quality.
- China's Position in the Global IVD Market
China's OEM Fecal H. Pylori AG Test Device illustrates the country's growing role in the global IVD market. With advanced technology and a focus on patient-centric solutions, China's contributions to the field are becoming increasingly significant. This test device is just one example of how Chinese manufacturers are setting new standards for diagnostic accuracy and affordability worldwide.
- The Importance of Non-invasive Diagnostic Tools
Non-invasive tests are crucial in modern medicine, providing patient comfort and ease of use. The China OEM Fecal H. Pylori AG Test Device exemplifies this trend by offering a reliable, non-invasive alternative for detecting H. pylori. As healthcare shifts towards more patient-friendly approaches, devices like these will be pivotal in meeting both clinical and patient demands.
- Challenges and Opportunities in H. Pylori Detection
The detection of H. pylori presents numerous challenges, particularly in ensuring accuracy and reliability. The China OEM Fecal H. Pylori AG Test Device addresses these challenges by offering a sensitive and specific diagnostic tool. By minimizing false results and enhancing user experience, this device offers substantial opportunities for improving H. pylori management globally.
- Future Trends in IVD Technology
As the IVD industry evolves, future trends are likely to focus on enhancing test accuracy and accessibility. The China OEM Fecal H. Pylori AG Test Device is a precursor to these advancements, combining rapid diagnostics with ease of use. By leveraging advancements in biotechnology, future IVD tools can be expected to provide even greater insights into patient health, paving the way for personalized medicine.
- Improving Healthcare Access with Rapid Diagnostics
Access to efficient diagnostic tools is crucial for enhancing healthcare delivery. The China OEM Fecal H. Pylori AG Test Device offers a quick and accessible option for diagnosing H. pylori, making it ideal for diverse settings. By promoting such technologies, we can ensure more patients receive timely and accurate diagnoses, regardless of their location.
- Patient-Centric Innovations in Medical Devices
Medical devices are increasingly being designed with patient experience in mind. The China OEM Fecal H. Pylori AG Test Device is an exemplar of this approach, providing a non-invasive, comfortable, and efficient diagnostic method. Such innovations ensure that patient needs are prioritized, leading to better satisfaction and outcomes.
- The Role of OEM Solutions in Healthcare
OEM solutions provide customized, high-quality products to meet specific healthcare needs. The China OEM Fecal H. Pylori AG Test Device is a testament to the value of OEM partnerships, delivering accurate diagnostic solutions that can be tailored to various healthcare environments. These collaborations are vital for advancing healthcare standards and meeting the unique challenges of medical diagnostics.
Image Description