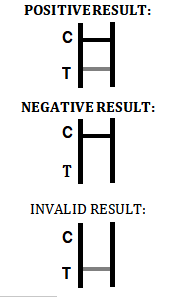China sFlT-1/PIGF Test for Pre-eclampsia Risk Assessment
Product Main Parameters
| Product Name | China sFlT-1/PIGF Test |
|---|---|
| Application | In vitro diagnosis of pre-eclampsia risk |
| Specimen | Maternal blood |
| Packing | 10 tests/box |
| Storage | 2-30°C |
Common Product Specifications
| Detection Method | Immunochromatographic assay |
|---|---|
| Testing Time | 15 minutes |
| Shelf Life | 12 months |
Product Manufacturing Process
The production of the China sFlT-1/PIGF Test involves several steps, including antigen preparation, coating of the test strip with receptors specific to sFlT-1 and PlGF, assembly of the test kits, and rigorous quality control to ensure accuracy and reliability. Highly sensitive materials and advanced biotechnological methods are employed to enhance the detection of protein levels, providing reliable results crucial for predicting pre-eclampsia risk. According to leading studies, efficient manufacturing processes directly correlate with the test’s high sensitivity and specificity, making it an indispensable tool for clinicians.
Product Application Scenarios
The China sFlT-1/PIGF Test is primarily used in prenatal clinics and hospitals where pregnant women are at risk of developing pre-eclampsia. It is an essential diagnostic tool for early detection, allowing healthcare providers to apply preventive measures and closer monitoring protocols. Studies confirm that early intervention significantly reduces complications associated with pre-eclampsia, improving maternal and fetal outcomes. The test is also valuable in managing high-risk pregnancies by identifying patients who require more intensive follow-up care.
Product After-sales Service
QL Biotech offers comprehensive after-sales support for the China sFlT-1/PIGF Test, including technical assistance, replacement of faulty products, and expert consultations. The customer service team is available to resolve any issues and provide guidance on product usage to ensure optimal performance and customer satisfaction.
Product Transportation
The China sFlT-1/PIGF Test is shipped under controlled conditions to maintain product integrity. Proper packaging ensures protection from temperature fluctuations and physical damage during transit. QL Biotech collaborates with trusted logistics partners to provide timely delivery to clients worldwide.
Product Advantages
- High sensitivity and specificity in determining pre-eclampsia risk.
- Simple and quick testing method yields results in 15 minutes.
- CE marked and manufactured under strict quality controls.
- Helps clinicians tailor pregnancy management plans effectively.
- Supports personalized medicine approaches in prenatal care.
Product FAQ
- What is the purpose of the China sFlT-1/PIGF Test?
The test is designed to evaluate the risk of pre-eclampsia in pregnant women by measuring the levels of sFlT-1 and PlGF proteins. By identifying an imbalance in these proteins, the test helps predict the likelihood of developing pre-eclampsia, allowing for timely intervention. - How is the test performed?
The test involves collecting a blood sample from the pregnant woman, which is then analyzed using the immunochromatographic assay method. Results are typically available within 15 minutes, facilitating quick decision-making in clinical settings. - What does a high sFlT-1/PIGF ratio indicate?
A high ratio suggests an increased risk of pre-eclampsia, as it indicates an anti-angiogenic state. Clinicians can use these results to devise a suitable management plan, including enhanced monitoring and preventive treatments. - What are the limitations of the test?
While the China sFlT-1/PIGF Test is highly specific, it should be used alongside clinical assessment and other diagnostic tools. It provides an indication of risk rather than a definitive diagnosis of pre-eclampsia. - Who should undergo this test?
The test is recommended for pregnant women who exhibit signs of possible pre-eclampsia or have factors that put them at elevated risk for the condition. It is also useful in evaluating women with high blood pressure or unusual ultrasound findings. - How reliable are the test results?
The China sFlT-1/PIGF Test is CE marked and has passed stringent quality control processes, ensuring high reliability and reproducibility of results in a clinical setting. - Can the test predict other pregnancy complications?
While primarily aimed at assessing pre-eclampsia risk, the test may indirectly help identify pregnancies requiring closer monitoring due to its insights into angiogenesis-related disorders. - Is the test widely accepted in clinical practice?
Yes, it is widely used in prenatal care due to its effectiveness in predicting pre-eclampsia, which is crucial for managing high-risk pregnancies and improving outcomes. - What should be done if a high risk is indicated?
If a high sFlT-1/PIGF ratio is detected, healthcare providers should consider heightened surveillance and possibly begin preventive treatments like low-dose aspirin, alongside lifestyle modifications. - What are the benefits of early detection?
Early detection of pre-eclampsia risk allows for timely interventions, significantly reducing potential complications and improving both maternal and fetal health.
Product Hot Topics
- Advancements in Pre-Eclampsia Diagnosis with China sFlT-1/PIGF Test
The introduction of the China sFlT-1/PIGF Test marks a significant advancement in prenatal care, providing a robust method for early detection of pre-eclampsia risk. As healthcare trends move towards personalized medicine, the test stands out by offering individualized risk assessment and facilitating tailored interventions. Its utility is highlighted in various clinical studies demonstrating its potential to improve pregnancy outcomes through early and precise risk stratification. - Reducing Maternal and Fetal Complications through Early Risk Assessment
The China sFlT-1/PIGF Test plays an essential role in identifying pregnancies at risk for pre-eclampsia, a condition notorious for causing maternal and fetal complications. By providing insights into angiogenic imbalances, the test supports timely management strategies that can mitigate risks significantly. Increased adoption of this test in clinical practice underscores its value in enhancing prenatal care standards. - Integrating China sFlT-1/PIGF Test in Routine Prenatal Care
Integrating the China sFlT-1/PIGF Test into routine prenatal evaluations enhances the ability of healthcare providers to monitor and manage pregnancies proactively. It aligns with global efforts to standardize pre-eclampsia screening, offering a reliable and efficient method for assessing risk, as advocated by leading obstetrics guidelines and research. - Precision Medicine in Obstetrics: China sFlT-1/PIGF Test as a Pioneer
The China sFlT-1/PIGF Test exemplifies precision medicine's application in obstetrics by providing targeted insights based on angiogenic profiles. This approach is increasingly recognized for its potential to optimize pregnancy care, enabling clinicians to implement specific interventions based on quantified risk levels, ultimately improving maternal and fetal health outcomes. - Global Trends and the Impact of China sFlT-1/PIGF Test on Pre-Eclampsia Management
As one of the leading tests for pre-eclampsia risk assessment, the China sFlT-1/PIGF Test is shaping global trends in prenatal care. Its impact is evident in various healthcare systems where it has become a standard component of risk assessment protocols, reflecting its effectiveness in enhancing early detection and management of pre-eclampsia. - Quality Assurance and Innovation in China sFlT-1/PIGF Test Development
QL Biotech's commitment to quality assurance and innovation in the development of the China sFlT-1/PIGF Test is evident in its robust manufacturing processes and strict quality controls. This dedication ensures the delivery of reliable and accurate testing instruments, supporting healthcare providers in offering the best possible care for their patients. - Implementing China sFlT-1/PIGF Test in High-Risk Pregnancy Clinics
High-risk pregnancy clinics are at the forefront of adopting the China sFlT-1/PIGF Test due to its proven efficacy in identifying women at increased risk for pre-eclampsia. This implementation allows for targeted monitoring and management strategies, which are critical in preventing severe pregnancy complications and ensuring patient safety. - Ensuring Accurate Results with China sFlT-1/PIGF Test: Best Practices
To achieve accurate and reliable results with the China sFlT-1/PIGF Test, healthcare professionals must adhere to best practices, including proper specimen collection and adherence to testing protocols. This vigilance helps maintain the test's high sensitivity and specificity, crucial for effective pre-eclampsia risk assessment. - Exploring the Role of China sFlT-1/PIGF Test in Personalized Prenatal Care
The China sFlT-1/PIGF Test is pivotal in the shift towards personalized prenatal care, offering insights that allow healthcare providers to tailor interventions based on individual risk profiles. This personalized approach is key to optimizing treatment plans and improving pregnancy outcomes. - The Future of Pre-Eclampsia Screening: Insights from China sFlT-1/PIGF Test
As research continues to explore new frontiers in prenatal care, the China sFlT-1/PIGF Test remains at the forefront of pre-eclampsia screening. Its ongoing integration into standard practices highlights its role in the future landscape of pregnancy management aimed at reducing pre-eclampsia morbidity and mortality rates.
Image Description