CMV IgG/IgM Test Cassette - Accurate & Fast Diagnosis
PRINCIPLE
The H. Pylori Ag Rapid Test Devic/Strip (Feces) is a non‐invasive lateral flow assay, rapid, precise and easy to perform.This test makes use of specific antibodies against H. Pylori antigen adsorbed onto a reactive membrane. If H. Pylori is present in stool specimen, the specific antigen is bound by the second antibody which is conjugated with colloidal gold particles. A generic antibody, fixed onto the reactive membrane, in shape of the band, is able to capture the second conjugated antibody, assuring the correctness of the test performance.
Product Detail
Brand: QL
Specimens: : Feces
Reading time:10 minutes.
Pack:25 T
STORAGE: 2‐30°C
KIT COMPONENTS(Device)
Individually packed Test Devices/Strips
Each test contains a strip with colored conjugates and reactive reagents pre-spreaded at the
corresponding regions
Tubes with buffer
Phosphate buffered saline and preservative, extract the samples
Package insert
For operation instruction
PROCEDURE
- Bring tests, specimens, buffer and/or controls to room temperature (15-30°C) before use.
1. Specimen collection and pre-treatment:
1) Unscrew and remove the dilution tube applicator. Be careful not to spill or spatter solution from the tube. Collect specimens by inserting the applicator stick into at least 3 different sites of the feces.
2) Place the applicator back into the tube and screw the cap tightly. Be careful not to break the tip of the dilution tube.
3) Shake the specimen collection tube vigorously to mix the specimen and the extraction buffer. Specimens prepared in the specimen collection tube may be stored for 6 months at -20°C if
not tested within 1 hour after preparation.
2. Testing
1) Remove the test from its sealed pouch, and place it on a clean, level surface. Label the test with patient or control identification. To obtain a best result, the assay should be performed within one hour.
2) Using a piece of tissue paper, remove the tip of the dilution tube. Hold the tube vertically and dispense 3 drops of solution into the sample well of the Test Device.
Avoid trapping air bubbles onto the sample pad, and do not drop any solution in observation window.
As the test begins to work, you will see color move across the membrane.
3. Wait for the colored band(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 20 minutes.
INTERPRETATION OF RESULTS
-
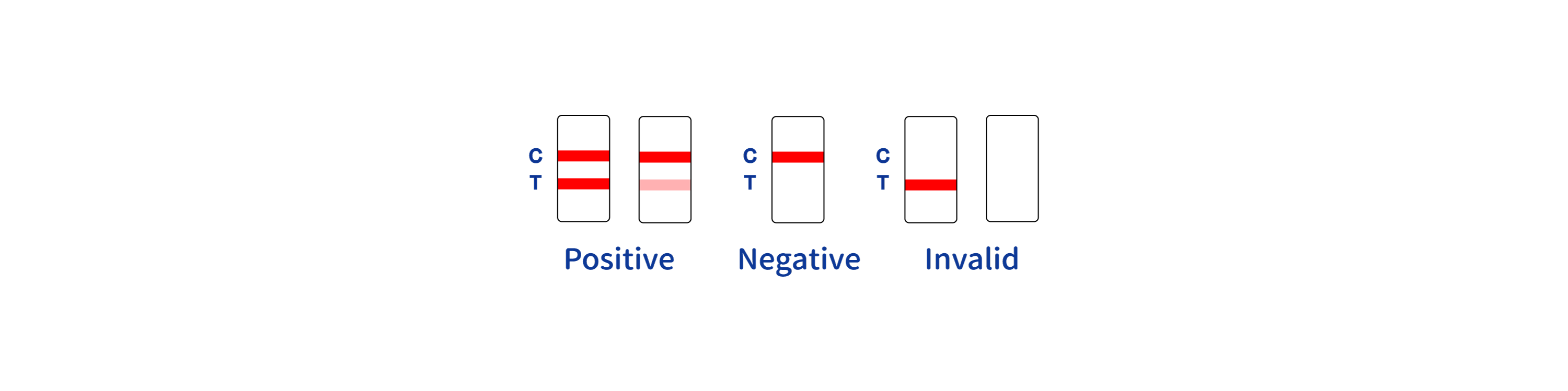
POSITIVE RESULT:
A colored band appears in the control band region (C) and another colored band appears in the T band region
NEGATIVE RESULT:
One colored band appears in the control band region (C). No band appears in the test band region (T)
INVALID RESULT:
Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor.
NOTE:
1. The intensity of the color in test region (T) may vary depending on the concentration of aimed substances present in the specimen. Therefore, any shade of color in the test region should be considered positive. Besides, the substances level can not be determined by this qualitative test.
2. Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
Our CMV IgG/IgM Test Cassette is based on the principle of the double-antigen sandwich method, a cutting-edge technology that enhances the accuracy of the test results. This method ensures that both IgG and IgM antibodies are detected simultaneously, providing a comprehensive understanding of the infection stage, which is crucial for effective treatment and management. The simplicity of the test procedure allows healthcare professionals to perform the test in various settings without the need for sophisticated laboratory equipment. Within just 15 to 20 minutes, results are ready, facilitating prompt decision-making in clinical practice. The significance of our product extends beyond its technical capabilities. In the landscape of infectious diseases, timely and accurate diagnosis plays a pivotal role in preventing the spread of infections and ensuring the well-being of communities. The CMV IgG/IgM Test Cassette stands as a testament to QL Biotech’s commitment to advancing healthcare solutions that are accessible, reliable, and efficient. With over 800 words of in-depth information and guidance provided with each product, users are equipped with the knowledge to not only conduct the test but also to understand its implications fully. Trust QL Biotech to be your partner in health, providing you with the tools you need to secure a healthier future.


