Dengue Combo Test Kit: Rapid HIV Ab & P24 Ag detection system
PRINCIPLE
Human Immunodeficiency Virus (HIV) Ab & P24 Ag Rapid Test Device (Whole Blood/Serum/Plasma) is a qualitative, membrane based immunoassay for the detection of antibodies to HIV 1&2 and P24 antigen in whole blood, serum or plasma. The membrane is precoated with recombinant HIV antigens to HIV 1&2 antibody and P24 antibody to HIV P24 antigen. During testing, the whole blood, serum or plasma specimen reacts with HIV antigen or P24 antibody coated particles in the test strip. The mixture then migrates upward on the membrane chromatographically by capillary action and reacts with recombinant HIV antigen or P24 antibody on the membrane in the test line regions. If the specimen contains antibodies to HIV 1 and/or HIV 2 and/or P24 antigen, a colored line will appear in the test line region indicating a positive result. If the specimen does not contain HIV 1 and/or HIV 2 antibodies and/or P24 antigen, no colored line will appear in the test line region indicating a negative result. To serve as a procedural control, a colored line will always appear in the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.
MATERIALS
Materials Provided
● Test devices ● Disposable specimen droppers
● Buffer ● Package insert
Materials Required But Not Provided
● Specimen collection containers ● Lancets (for fingerstick whole blood only)
● Centrifuge (for plasma only) ● Timer
● Disposable heparinized capillary tubes and dispensing bulb (for fingerstick whole blood only)
DIRECTIONS FOR USE
-
● Test devices
● Disposable specimen droppers
● Buffer
● Package insert
Materials Required But Not Provided
● Specimen collection containers
● Lancets (for fingerstick whole blood only)
● Centrifuge (for plasma only)
● Timer
● Disposable heparinized capillary tubes and dispensing bulb (for fingerstick whole blood only)
PERFORMANCE CHARACTERISTICS
Sensitivity
Human Immunodeficiency Virus (HIV) Ab & P24 Ag Rapid Test Device (Whole
Blood/Serum/Plasma) has been tested by anti-HIV 1 low titer performance panel, anti-HIV 2 performance panel and anti-HIV 1 seroconversion panel (Boston Biomedica, Inc.). And it has also been compared with leading commercial EIA HIV test on clinical specimens. The results show that
HIV 1&2 Human Immunodeficiency Virus Rapid Test Device (Whole Blood/Serum/Plasma) is very sensitive to HIV 1 and/or HIV 2 antibodies.
Specificity
The specificity of the test is comparable to a leading commercial HIV EIA test. Human Immunodeficiency Virus (HIV) Ab & P24 Ag Rapid Test Device (Whole Blood/Serum/Plasma) is highly specific for anti-HIV 1 and/or HIV 2 compared to a leading commercial HIV EIA test.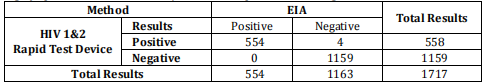
Relative Sensitivity: 99.9% (99.3%-100.0%)*Relative Specificity: 99.6% (99.1%-99.9%)*
Relative Accuracy: 99.8% (99.4%-99.9%)* * 95% Confidence Interval
Precision
Intra Assay
Within-run precision has been determined by using 15 replicates of three specimens: a negative, a low positive and a high positive. The negative, low positive and high positive values were correctly identified 99.5% of the time.
Inter-Assay
Between-run precision has been determined by 15 independent assays on the same three specimens: a negative, a low positive and a high positive. Three different lots of Human
Immunodeficiency Virus (HIV) Ab & P24 Ag Rapid Test Device (Whole Blood/Serum/Plasma) have been tested using negative, low positive and high positive specimens. The specimens were correctly identified 99.5% of the time.
This kit is more than just a rapid test device. It’s a crucial tool for disease control, patient care, and medical advancement. Invest in the Dengue Combo Test Kit - your solution for accurate, swift, and reliable HIV detection. Please note that the Dengue Combo Test Kit is designed for laboratory use and should be used by trained personnel to ensure accurate results. Always consult a healthcare professional for interpretation of test results.


