Easy Operate Dengue IgG/IgM Test: Quick RSV Antigen Detection Device by QL Biotech
PRINCIPLE
The RSV Ag Rapid Test device (Swab) detects RSV antigens through visual interpretation of color development on the strip. RSV antibodies are immobilized on the test region of the membrane respectively. During testing, the extracted specimen reacts with anti- RSV antibodies conjugated to colored particles and precoated onto the sample pad of the test. The mixture then migrates through the membrane by capillary action and interacts with reagents on the membrane. If there is sufficient RSV antigens in the specimen, colored band will form at the according test region of the membrane. The presence of a colored band in the test region indicates a positive result for the particular viral antigens, while its absence indicates a negative result. The appearance of a colored band at the control region serves as a procedural control, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
Product Detail
-
-
Brand: QL
Specimens: : Nasopharyngeal swabs/ Nasal swab
Reading time:10 minutes.
Pack:20 T
STORAGE: 2‐30°C
KIT COMPONENTS(Device)
Individually packed Test Devices
Extraction solution
Extraction tubes
Sterile nasal swabs
Package insert
-
PROCEDURE
- Bring tests, specimens, and/or controls to room temperature (15-30°C) before use.
1. Remove the test from its sealed pouch, and place it on a clean, level surface. Label the Strip with patient or control identification. For best results, the assay should be performed within one hour.
2. Gently mix Extraction reagent solution.
For bottle buffer: Add 5 drops (about 200ul) of the Extraction Solution into the Extraction tube.
For single buffer: transfer all buffer (about 200ul) into the extraction tube.
3. Place the patient swab specimen into the Extraction Tube. Roll the swab at least 10 times while pressing the swab against the bottom and side of the Extraction Tube. Roll the swab head against the inside of the Extraction Tube as you remove it. Try to release as much liquid as possible. Dispose of the used swab in accordance with your biohazard waste disposal protocol.
4. Put the strip into the extraction test tube and then start the timer.
5. As the test begins to work, color will migrate across the membrane. Wait for the colored band(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 20 minutes.
RESULTS
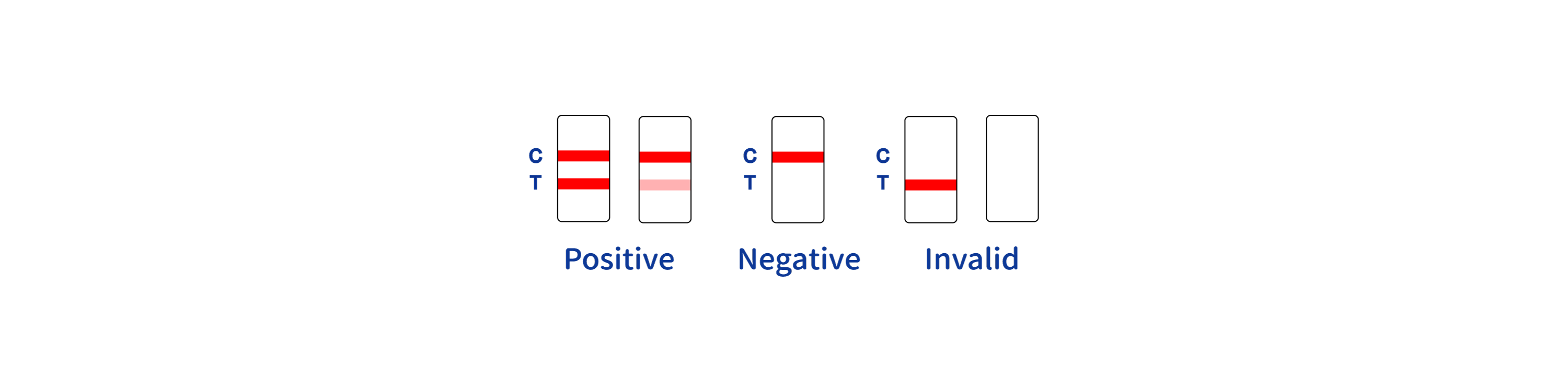
- POSITIVE RESULT:
- A colored band appears in the control band region (C) and another colored band appears in the T band region
- NEGATIVE RESULT:
- One colored band appears in the control band region (C). No band appears in the test band region (T)
- INVALID RESULT:
- Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor
- NOTE:
- 1.The intensity of the color in test region (T) may vary depending on the concentration of aimed substances present in the specimen. Therefore, any shade of color in the test region should be considered positive. Besides, the substances level can’t be determined by this qualitative test.
- 2.Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
Furthermore, the inclusion of the Easy Operate Dengue IgG/IgM Test in our RSV Ag Rapid Test Device ensures an uncomplicated testing process. Simplified operation, coupled with rapid result generation, adds to the device's appeal for clinical practices requiring quick and accurate RSV detection. Accompanied by our comprehensive user manual, the test device is designed to minimize human error. In conclusion, the RSV Ag Rapid Test Device from QL Biotech, powered by the Easy Operate Dengue IgG/IgM Test, is an integral tool in swift and precise RSV detection. It is a testament to our commitment to contributing quality, efficiency, and reliability to the medical diagnostic field. Upgrade your lab testing regimen with our user-friendly and rapid RSV antigen detection tool today.


