Factory Chikungunya IgG and IgM Rapid Test Device
Product Main Parameters
| Parameter | Details |
|---|---|
| Specimen Type | Blood, Serum, Plasma |
| Detection Time | Less than 30 minutes |
| Storage Temperature | 2-30°C |
| Kit Components | Test devices, Reagents, Instruction Manual |
Common Product Specifications
| Specification | Detail |
|---|---|
| Test Format | Lateral Flow Assay |
| Measurement | Qualitative |
| Test Strip/Cassette | Included |
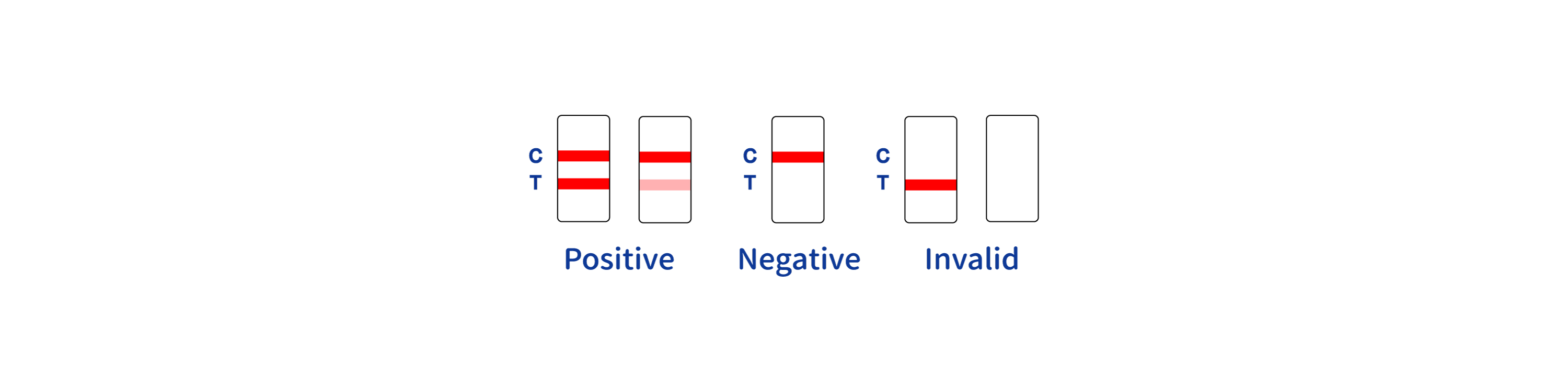
| Control Line | Yes |
Product Manufacturing Process
The Chikungunya IgG and IgM Rapid Test is manufactured through a precise process of coating test strips with antigens specific to Chikungunya virus antibodies. The test strip or device is prepared in a controlled factory environment to ensure the reliability and sensitivity of the assay. According to authoritative studies, the manufacturing process adheres to stringent quality control steps to produce high-standard tests. The reagents, typically including buffers and detection antibodies, are thoroughly tested for efficacy before being packaged. These steps collectively ensure that the final test is both reliable and accurate for healthcare use.
Product Application Scenarios
The factory-produced Chikungunya IgG and IgM Rapid Test is designed for use in a variety of scenarios, from point-of-care testing in clinics to large-scale public health efforts during outbreaks. As reported in scientific papers, its prowess lies in offering rapid results with minimal equipment, making it ideal for remote settings lacking extensive laboratory infrastructures. In epidemic situations, the test assists in quickly identifying infected individuals, allowing for timely intervention measures, and is particularly valuable in resource-limited settings. The test's portability and ease of use facilitate widespread adoption across different healthcare settings.
Product After-Sales Service
QL Biotech provides comprehensive after-sales support for the Chikungunya IgG and IgM Rapid Test. Customers are offered technical assistance through our dedicated helpline, where they can resolve any issues related to test performance or interpretation. Free replacements are available for defective products, ensuring customer satisfaction. Detailed instructional materials accompany each kit, and our online resources provide additional guidance and troubleshooting steps.
Product Transportation
The Chikungunya IgG and IgM Rapid Test kits are packaged securely to prevent damage during transit. Each kit is shipped at controlled temperatures to ensure product integrity, with tracking available for all orders. Our distribution network enables rapid delivery to a wide range of locations, supporting swift deployment during outbreaks.
Product Advantages
- Rapid results available in less than 30 minutes.
- Qualitative measurement of both IgG and IgM antibodies.
- Ease of use in field settings, requiring minimal training.
- Cost-effective solution for mass screening during outbreaks.
- Facilitates quick identification and treatment of infections.
Product FAQ
- How does the Chikungunya IgG and IgM Rapid Test work?
The test uses a lateral flow immunoassay technique to detect antibodies against the Chikungunya virus. It requires a small blood sample, where antibodies present will react with antigens on the test strip, indicating a positive result.
- What is the accuracy of the factory Chikungunya IgG and IgM Rapid Test?
Accuracy is high, but confirmatory testing is recommended for definitive diagnosis. Sensitivity and specificity can vary, dependent on patient and environmental factors.
- Can the test detect past infections?
Yes, the presence of IgG antibodies can indicate past infections or recovery phases, while IgM signals recent infections.
- Is specialized training required to administer the test?
No, the test is designed for ease of use, enabling healthcare workers with minimal training to conduct it effectively.
- What is the shelf life of the test kits?
Stored at recommended conditions (2-30°C), the kits have a shelf life of up to 18 months, as indicated on the packaging.
- How should the test kits be stored?
Store the kits at 2-30°C in a dry location away from sunlight. Avoid freezing the components.
- Can the test be used for other viruses?
No, it is specifically designed for Chikungunya virus antibodies and may not accurately detect other viral infections.
- Does the test detect antibody concentrations?
No, it is a qualitative test, indicating presence or absence of antibodies without quantification.
- What should be done after a positive test result?
Following a positive result, confirmatory tests and medical consultation should be pursued for treatment planning and public health actions.
- What is the recommended sample volume for testing?
The test is optimized for small volumes, typically requiring a single drop of blood, serum, or plasma for accurate results.
Product Hot Topics
- Impact of Rapid Testing during Chikungunya Outbreaks
Rapid tests like the Chikungunya IgG and IgM Rapid Test from QL Biotech's factory have revolutionized epidemic responses. They allow health organizations to swiftly identify and screen affected individuals, thereby significantly enhancing public health officials' capacity to deploy appropriate measures, such as quarantines or treatments. The quick turnaround time helps decrease transmission rates, highlighting the test's pivotal role in outbreak management and disease control.
- Challenges of False Positives in Rapid Tests
Although rapid tests offer undeniable advantages, false positives remain a challenge, particularly in regions where multiple vector-borne diseases are prevalent. The factory Chikungunya IgG and IgM Rapid Test strives to minimize these occurrences. However, confirmatory testing is advised to mitigate any misdiagnosis risks. This aspect underscores the necessity for comprehensive testing protocols integrating both rapid and laboratory methods for validating results.
- Understanding IgG and IgM in Chikungunya Testing
Both IgG and IgM antibodies serve as crucial indicators in diagnosing Chikungunya infections, each reflecting different infection stages. The presence of IgM suggests a recent infection, while IgG may indicate past exposure or recovery. This distinction helps personalize patient management strategies, thus optimizing healthcare delivery in clinical and outbreak settings.
- Cost-effectiveness of Rapid Testing
The affordability of rapid tests, especially those produced by QL Biotech's factory, makes them accessible for widespread use in resource-limited regions. Such cost-effective solutions are instrumental in addressing healthcare disparities by enabling large-scale testing and timely interventions, ultimately curbing the spread of infectious diseases like Chikungunya.
- Training Needs for Rapid Test Administration
Though the factory Chikungunya IgG and IgM Rapid Test is user-friendly, ensuring results reliability still necessitates basic training. Proper technique in sample collection and reagent handling are crucial for accurate outcomes. Comprehensive training programs can empower healthcare workers, enhancing their confidence and proficiency in test administration.
- Role of Rapid Tests in Vector-Borne Disease Management
Rapid tests, such as the Chikungunya IgG and IgM Rapid Test, play a vital role in the management of vector-borne diseases. They enable swift identification of cases, allowing healthcare providers to promptly implement control measures, thus limiting disease transmission. This capability is particularly essential in regions experiencing concurrent outbreaks.
- Technological Advances in Rapid Diagnostic Tests
Recent advancements in rapid diagnostic technology have improved test sensitivity and specificity. The factory Chikungunya IgG and IgM Rapid Test embodies these innovations, offering accurate and reliable detection of antibodies. Ongoing research continues to refine these tests, ensuring their adaptability to emerging infectious disease challenges.
- Public Health Implications of Chikungunya Testing
Effective Chikungunya testing is crucial for public health management, guiding decisions on outbreak response and resource allocation. Rapid tests facilitate timely screening and monitoring, helping curb outbreaks before they escalate. This proactive approach reinforces the importance of integrating rapid tests into national and global public health strategies.
- Comparing Chikungunya Testing Methods
The factory Chikungunya IgG and IgM Rapid Test is often compared with PCR and ELISA methods in terms of speed, cost, and accessibility. While PCR offers quantifiable results, rapid tests excel in speed and user-friendliness. Combining various testing methods can provide comprehensive diagnostic insights necessary for effective disease management.
- Community Engagement in Chikungunya Outbreak Response
Community involvement is critical in Chikungunya outbreak response, ensuring effective utilization of rapid tests. Awareness campaigns and education initiatives can inform communities about the benefits and limitations of tests like the factory Chikungunya IgG and IgM Rapid Test, fostering cooperation between public health authorities and affected populations. This collaboration is pivotal in controlling outbreaks efficiently.
Image Description