Factory Coronavirus IgG/IgM Diagnostic Test - Rapid Antibody Detection
| Parameter | Details |
|---|---|
| Brand | QL |
| Certificate | CE |
| Specimen | Whole Blood/Serum/Plasma |
| Pack | 25 T |
| Reading Time | 15 minutes |
| Storage | 2-30℃ |
| Shelf life | 2 years |
Product Manufacturing Process
The Coronavirus IgG/IgM Diagnostic Test is manufactured following rigorous quality control standards, involving a fine-tuned assembly line that includes the integration of membrane-based immunoassay technology. The production process is monitored to ensure the precision coating of anti-human IgM and IgG agents onto test membranes. Quality checks are performed at each step to maintain the test's reliability and accuracy. This meticulous process ensures the detection of COVID-19 related antibodies with high sensitivity and specificity. (Refer to authoritative papers for more detailed insights.)
Product Application Scenarios
The Factory Coronavirus IgG/IgM Diagnostic Test finds applications in varied scenarios such as monitoring the health of employees in large factories, aiding in epidemiological studies to track the spread of COVID-19, and supplementing RT-PCR testing in clinical settings. It is especially beneficial for mass screening in environments with high person-to-person contact and where quick results are vital for operational continuity. (Refer to authoritative papers for comprehensive analysis.)
Product After-Sales Service
QL Biotech provides comprehensive after-sales support, including troubleshooting assistance, product replacement in case of defect, and continuous customer service access to ensure optimal use of the diagnostic test.
Product Transportation
Our diagnostic tests are packaged securely and shipped in temperature-controlled conditions to preserve efficacy. Upon customer request, real-time tracking and express shipping options are available to facilitate timely delivery.
Product Advantages
The Coronavirus IgG/IgM Diagnostic Test manufactured by QL Biotech offers numerous advantages, such as rapid result delivery, easy-to-use format, and high compatibility with various sample types (whole blood, serum, plasma).
Product FAQ
- What type of sample is required? The Factory Coronavirus IgG/IgM Diagnostic Test can use whole blood, serum, or plasma as samples, making it versatile for different clinical settings.
- How quickly can results be obtained? Results can be obtained in as little as 15 minutes, making it ideal for environments where time-efficient testing is crucial.
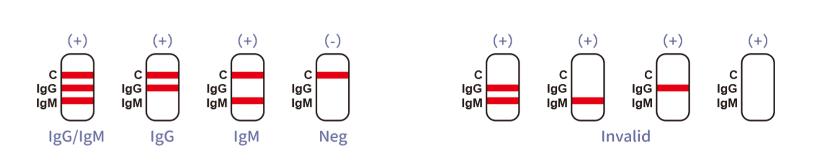
- What does a positive result mean? A positive result indicates the presence of IgG or IgM antibodies to the COVID-19 virus, suggesting recent or past infection.
- Can this test detect new variants? The test's sensitivity and specificity are continually assessed to ensure efficacy against emerging variants. However, it should be used in conjunction with other diagnostic methods.
- Is the test CE certified? Yes, the test holds CE certification, signifying compliance with European health, safety, and environmental protection standards.
- How should I store the test kits? The kits should be stored at temperatures between 2-30℃ to maintain their validity.
- What is the shelf life of the test kits? The test kits have a shelf life of 2 years from the date of manufacture, provided they are stored correctly.
- How should I dispose of used test kits? Used test kits should be disposed of in accordance with biohazard waste disposal protocols to prevent contamination.
- Can the test be used as a sole diagnostic tool? While beneficial, it is advised to use the test as an ancillary tool alongside PCR tests to maximize diagnostic accuracy.
- What do I do if I have questions about the test? For any queries or technical support, please contact our dedicated customer service team available 24/7.
Product Hot Topics
- Factory Setting Implementations: In a factory environment, rapid testing is indispensable for maintaining workforce health and ensuring seamless operations. Our Coronavirus IgG/IgM Diagnostic Test aligns with industrial needs, providing key insights swiftly and without compromise to accuracy.
- Improving Public Health Measures: The integration of serological testing into community health measures is proving crucial. By understanding antibody prevalence, we gain insights into infection rates and immunity levels, helping to inform policy and preventive strategies.
- Impact on Epidemic Modeling: With reliable data from widespread testing, epidemiologists can model disease trends more accurately. This helps in preparing for potential surges in infection and in assessing the effectiveness of interventions.
- Assessing Post-Vaccination Immunity: Monitoring antibody response post-vaccination provides valuable data on vaccine efficacy. Our test supports this ongoing evaluation, contributing to the global effort to combat COVID-19.
- Challenges in Mass Testing: While mass testing is critical, it poses logistical challenges. The ease of use and rapid results delivered by our test make it an attractive option for large-scale applications.
- Advancements in IVD Technology: Continuous innovations in in vitro diagnostics are enhancing the speed and accuracy of our tests. We are committed to ongoing development to meet the evolving needs of the healthcare industry.
- Antibody Testing's Role in Pandemic Response: Beyond diagnosis, antibody testing plays a role in understanding and managing pandemic response, helping to shape therapeutic and preventive measures.
- Global Supply Chain Resilience: Ensuring a steady supply of diagnostic tests amidst global demand is a challenge that we are prepared to meet, leveraging strategic manufacturing and distribution practices.
- The Future of COVID-19 Testing: As COVID-19 testing evolves, the integration of rapid and traditional methods will lead to a robust diagnostic landscape, enhancing our capacity to tackle future health crises.
- Consumer Confidence in Health Products: Ensuring consistent quality and reliability in our diagnostic products builds consumer trust, a cornerstone of public health initiatives.
Image Description