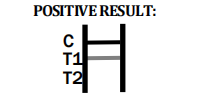
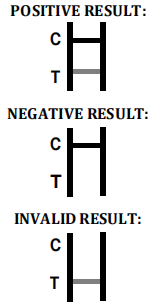Factory Direct Toxoplasma Gondii Test Kit
Product Main Parameters
| Specimen Type | Whole Blood/Serum/Plasma |
|---|---|
| Reading Time | 15 minutes |
| Storage Temperature | 2‐30°C |
| Pack Size | 25 Tests |
Common Product Specifications
| Test Principle | Qualitative membrane‐based immunoassay |
|---|---|
| Kit Components | Test devices, pipettes, buffer, insert |
Product Manufacturing Process
According to authoritative studies, the production of Toxoplasma gondii test kits involves precise processes to ensure the reliability and accuracy of results. Key steps include sourcing high-quality materials to ensure the integrity of the test, utilizing advanced technology for the formulation of reactive agents, and rigorous testing of control measures to uphold quality standards. The conclusion drawn from recent papers is that factory-standardized processes, combined with quality control protocols, significantly enhance the efficacy of diagnostic reagents.
Product Application Scenarios
The Toxoplasma gondii test kit is used primarily in clinical laboratories and healthcare settings to diagnose and manage infections. Research papers indicate that rapid and accurate testing is crucial for preventing complications, particularly in pregnant women and immunosuppressed individuals. These kits facilitate quick decision-making by healthcare providers, enabling prompt and effective treatment interventions. The takeaway from literature is that the integration of reliable testing tools in medical practice significantly improves patient outcomes and public health management.
Product After-Sales Service
- 24/7 Customer Support
- Comprehensive User Manual
- Product Warranty: One-year
- Free Replacement for Defective Items
Product Transportation
Our distribution network ensures timely delivery of Toxoplasma gondii test kits from the factory to your doorstep. Each package is carefully handled to preserve the integrity of the test components during transit, maintaining optimal performance upon arrival.
Product Advantages
- Accurate and Reliable Results
- User-Friendly Design
- Minimal Training Required
- Rapid Testing Process
Product FAQ
- Q: What is the primary use of this test kit?
A: The Toxoplasma gondii test kit is designed for diagnosing T. gondii infections in whole blood, serum, or plasma specimens, facilitating quick and effective clinical decision-making. - Q: How should I store the test kits?
A: Store the kits at a temperature between 2‐30°C. Avoid exposing them to direct sunlight and extreme temperatures to maintain test accuracy. - Q: Is the test kit suitable for home use?
A: The test kit is intended for professional use in clinical environments, where accurate diagnosis and interpretation can be performed by trained personnel. - Q: Can this test detect past infections?
A: The kit is designed to detect active T. gondii infections. For past infections, other diagnostic methods such as serology may be required to detect IgG antibodies. - Q: How is the accuracy of the test verified?
A: The test undergoes rigorous quality control and calibration procedures during manufacturing at the factory to ensure high accuracy and reliability.
Product Hot Topics
Is Toxoplasma Gondii Testing Essential for Pregnant Women?Toxoplasma gondii infections can pose significant risks to unborn children if contracted during pregnancy. Testing with reliable kits from a trusted factory like QL Biotech can aid in timely diagnosis, reducing the risk of severe complications. Utilizing accurate tests ensures that any necessary interventions are taken swiftly, such as potential treatments to prevent fetal impacts. Hence, implementing Toxoplasma gondii testing in prenatal care is crucial for safeguarding maternal and fetal health.
Advancements in Factory-Made Toxoplasma Gondii Diagnostic ToolsThe development of more sophisticated and reliable Toxoplasma gondii diagnostic kits has been a significant focus for factories like QL Biotech. These advancements ensure that tests are not only quicker and easier to administer but also more accurate in detecting the infection at various stages. Continuous research and development efforts are pivotal in enhancing testing protocols, making them indispensable in managing and controlling Toxoplasma infections effectively in diverse healthcare settings.
Image Description