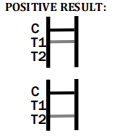
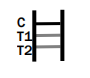
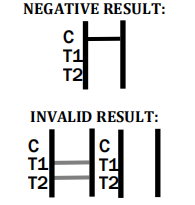
Factory-Made Disposable Syphilis Rapid Test Device
Product Main Parameters
| Parameter | Details |
|---|---|
| Format | Rapid Test Cassette |
| Sample Type | Whole Blood, Serum, Plasma |
| Reading Time | 10-20 minutes |
| Shelf Life | 12 months |
| Storage | 2-30°C |
Common Specifications
| Specification | Details |
|---|---|
| Pack Size | 25 Tests |
| Sensitivity | >99% |
| Specificity | >99% |
Product Manufacturing Process
The manufacturing process of the factory's Disposable Syphilis Rapid Test Device involves several key stages. Initially, recombinant proteins specific to Treponema pallidum are produced and purified through advanced chromatography techniques. The reagents used in the test strips are prepped by incorporating colloidal gold conjugation. The assembly process includes the integration of these reagents onto nitrocellulose membranes, which are precisely cut and assembled into plastic cassettes. Each unit is individually sealed to preserve sterility. Rigorous quality control measures ensure the reliability and accuracy of every test.
Product Application Scenarios
The Disposable Syphilis Rapid Test Device is highly suited for various application scenarios due to its portability and ease of use. In clinical settings, it facilitates rapid point-of-care testing, allowing healthcare professionals to make immediate diagnostic decisions. In remote or resource-limited areas, its user-friendly operation and minimal equipment requirements make it invaluable for field screenings and outreach programs. The factory's device is also beneficial during public health campaigns, enhancing STI control by providing quick, on-site testing solutions.
Product After-Sales Service
- Technical Support: Available 24/7 to assist with any inquiries or issues.
- Replacement Policy: Defective products can be replaced within a specified warranty period.
- Customer Training: Offers online tutorials and training workshops for proper usage.
Product Transportation
Our Disposable Syphilis Rapid Test Devices are shipped from the factory securely packed to prevent damage during transit. We utilize temperature-controlled logistics to maintain product integrity, and offer global shipping options with tracking facilities to ensure timely delivery.
Product Advantages
- Speed: Results available in minutes.
- Portability: Compact and easy to transport.
- Cost-Effective: Minimizes equipment and training costs.
- Convenience: Simple to administer in various settings.
- Reliability: High sensitivity and specificity.
Product FAQ
- What is the shelf life of the Disposable Syphilis Rapid Test Device? The shelf life is 12 months from the date of manufacture, provided it is stored as per the recommended conditions from the factory.
- Can the test differentiate between active and past infections? While it effectively detects antibodies, further laboratory tests are advised to distinguish between active and past infections.
- What specimen types are compatible with the test? The test is compatible with whole blood, serum, and plasma specimens.
- How should the test be stored? The test should be stored at temperatures between 2°C and 30°C, away from direct sunlight and moisture.
- Is it necessary to confirm a positive result? Yes, confirmation with more specific tests is recommended to ensure accurate diagnosis.
- How many tests are included in a pack? Each pack contains 25 individually wrapped test devices.
- What is the time frame for reading the test result? Results should be read within 15 minutes, and no later than 20 minutes to ensure accuracy.
- Can the test be used in remote areas? Yes, its portability and ease of use make it suitable for remote and field settings.
- What is the accuracy rate of the test? The factory reports the test has an accuracy rate exceeding 99%.
- What training is required to use the test? Minimal training is required; however, guidelines provided by the factory should be followed carefully.
Product Hot Topics
- How does the factory ensure the quality of each Disposable Syphilis Rapid Test Device? At our factory, we incorporate stringent quality control measures at every stage of production. From the purification of recombinant proteins to the final assembly of test cassettes, every step is monitored and verified for accuracy and consistency. Batch testing is conducted to confirm the performance standards before market release, ensuring the precision and reliability that healthcare professionals depend on for rapid diagnostics.
- Portability in Diagnostics: The Role of the Factory's Rapid Test Device The portability of our factory-produced Disposable Syphilis Rapid Test Device marks a significant advancement in diagnostic accessibility. Designed for ease of transport and quick deployment, these devices are transforming how we approach STI screenings in varied environments. From urban clinics to rural health camps, the ability to test on-site reduces logistical challenges and brings immediate results, empowering healthcare providers to deliver timely interventions.
Image Description