Factory Rubella IgG/IgM Rapid Test Device
Product Main Parameters
| Parameter | Details |
|---|---|
| Test Type | Rapid Chromatographic Immunoassay |
| Specimens | Serum/Plasma |
| Reading Time | 20 minutes |
| Storage | 2-30°C |
Common Product Specifications
| Specification | Details |
|---|---|
| Pack | 25 T |
| Components | Test devices, pipettes, buffer, insert |
Product Manufacturing Process
The Rubella IgG/IgM rapid test device is manufactured under stringent quality controls at the QL Biotech factory. The process involves the application of advanced chromatographic techniques to ensure the sensitivity and specificity of antibody detection. Based on studies, the membrane-based immunoassay format is chosen for its rapid performance and reliability, suitable for point-of-care testing environments.
Product Application Scenarios
The Rubella IgG/IgM rapid test is vital in clinical settings, especially in prenatal care to assess immunity and risk of rubella. Its application extends to public health programs focusing on rubella outbreak control. Literature underscores its importance in timely diagnosis and prevention, ensuring necessary interventions are implemented to prevent congenital infections.
Product After-Sales Service
QL Biotech provides comprehensive after-sales support, including product training, technical assistance, and readily available expert consultation to ensure optimal use.
Product Transportation
Products are packed securely in climate-controlled conditions to maintain their integrity during transit, ensuring they reach the destination in perfect testing condition.
Product Advantages
This rapid test device offers quick results aiding in faster clinical decision-making. Its ease of use, coupled with minimal training requirements, makes it suitable for various healthcare settings.
Product FAQ
What specimens are needed for this test?
The test requires serum or plasma specimens for accurate results, aligning with factory standards for sample processing.
How long do results take?
Results appear within 20 minutes, providing quick feedback for Rubella IgG/IgM presence.
What is the storage condition for the test kit?
Store the kit between 2-30°C as recommended by factory guidelines to ensure longevity and efficacy.
Is this test qualitative or quantitative?
It is a qualitative test designed to indicate presence or absence of Rubella IgG/IgM antibodies.
Can this test differentiate between IgG and IgM?
The test distinguishes Rubella IgG from IgM, aiding in understanding immunity status or recent infection exposure.
What is the shelf life of the product?
Typically, the shelf life is one year under recommended storage conditions, maintained by factory quality controls.
Can this test be used for prenatal care?
Yes, it is widely used in prenatal screenings to assess Rubella immunity, as supported by factory-developed protocols.
What steps are involved in the testing procedure?
The procedure involves sample and buffer addition followed by visual interpretation of results after 20 minutes.
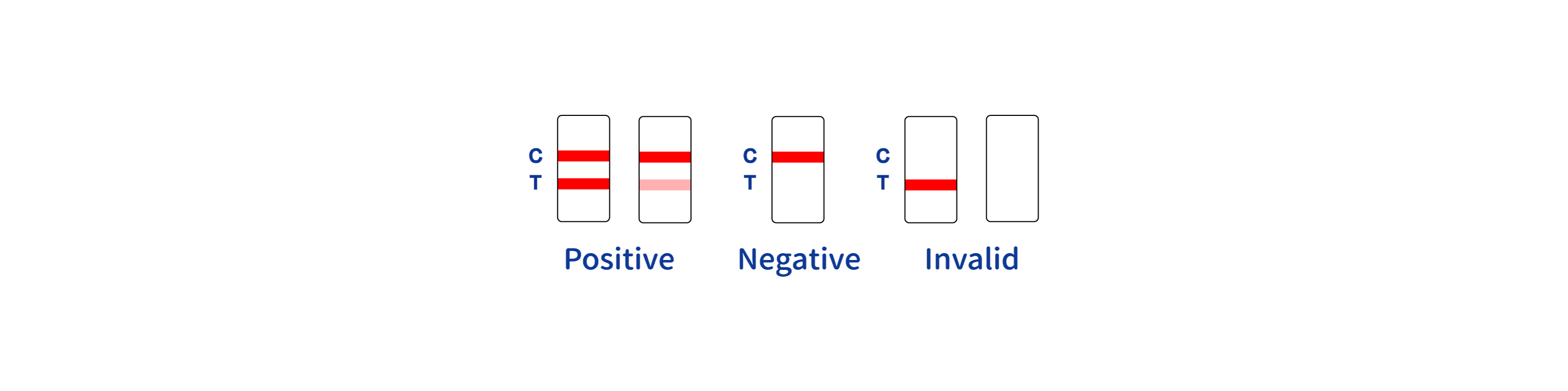
What if the control line does not appear?
In such cases, the test is invalid and should be repeated; consistent errors should be reported to the factory for a resolution.
How does this test contribute to public health?
By providing rapid results for Rubella IgG/IgM detection, the test assists in outbreak management and prevention initiatives.
Product Hot Topics
Understanding Rubella IgG/IgM Tests
The Rubella IgG/IgM tests play a crucial role in determining immunity and recent exposure to the rubella virus. The factory-developed test is a reliable option for both healthcare providers and public health initiatives, serving as a frontline tool in managing rubella risks, particularly in pregnant women.
The Role of Rapid Tests in Modern Diagnostics
Rapid tests, like the Rubella IgG/IgM test produced by QL Biotech's factory, offer convenience and speed in diagnosis, making them invaluable in clinical settings where time is of the essence. Their ease-of-use and minimal equipment requirements further enhance their applicability across diverse medical scenarios.
Impact of Rubella Immunity Assessment
Assessing Rubella IgG/IgM levels is vital for preventing congenital rubella syndrome. The QL Biotech factory's reliable test aids in identifying immunization needs, thus contributing to broader public health goals.
QA Standards in Test Manufacturing
Quality assurance is paramount in the production of diagnostic tests. At the QL Biotech factory, rigorous QA protocols ensure that each Rubella IgG/IgM test meets high standards of accuracy and reliability, essential for clinical and public health use.
Technical Advances in Rubella Testing
With continuous advancements in technology, the Rubella IgG/IgM test from QL Biotech's factory integrates the latest in chromatographic techniques, ensuring precision in antibody detection critical for patient care.
FAQs Around Rubella Testing
Common queries about Rubella IgG/IgM tests often involve their accuracy, interpretation, and procedural guidelines. The comprehensive FAQ section provided by QL Biotech's factory addresses these concerns, ensuring users are well-informed and confident in test application.
Ensuring Accurate Results in Rapid Testing
Accurate results are the cornerstone of effective diagnostics. At the factory level, QL Biotech ensures each Rubella IgG/IgM device is calibrated for precision, providing healthcare professionals with dependable tools for patient assessment.
Rubella Testing in Prenatal Care
The role of Rubella IgG/IgM testing in prenatal care is undeniable. Tests produced by QL Biotech's factory help in safeguarding fetal health by assessing maternal immunity and mitigating rubella risks during pregnancy.
Factory's Role in Test Reliability
The reliability of diagnostic tests often hinges on the manufacturing processes. The QL Biotech factory's commitment to quality ensures each Rubella IgG/IgM test is produced with the utmost care, meeting global standards and enhancing user confidence.
Future of Infectious Disease Diagnostics
With increasing emphasis on rapid diagnostics, the future sees a significant role for tests like the Rubella IgG/IgM produced at the QL Biotech factory. Their quick turn-around time and accuracy make them indispensable in modern healthcare and disease control efforts.
Image Description