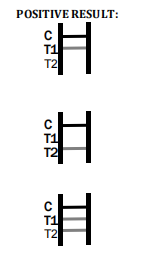
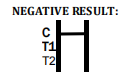
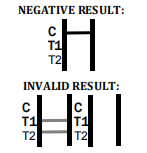
Factory Tuberculosis Blood Test Device – Quick & Reliable
Product Main Parameters
| Brand | QL Biotech |
| Specimens | Whole Blood/Serum/Plasma |
| Reading Time | 15 minutes |
| Pack | 25 Tests |
| Storage | 2-30°C |
Common Product Specifications
| Test Device | Individually packed with colored conjugates |
| Disposable Pipettes | Included for specimen addition |
| Buffer | Phosphate buffered saline with preservative |
| Package Insert | Instructions for use |
Product Manufacturing Process
The manufacturing of the Tuberculosis Blood Test device involves the precise integration of specific TB antigens within a lateral flow immunoassay framework. According to authoritative studies, lateral flow tests like this utilize nitrocellulose membranes and conjugate pads that hold antigens labeled with a detection conjugate. These tests undergo rigorous quality control to ensure the specificity and sensitivity required for accurate TB detection. The controlled manufacturing environment ensures that each device meets the high standards expected from QL Biotech's factory, delivering reliable and consistent performance in clinical applications.
Product Application Scenarios
Tuberculosis Blood Test devices are critical in healthcare settings that focus on infectious disease management, particularly in regions with high TB incidence. According to authoritative sources, these tests are ideal for initial TB screenings in clinics and hospitals, aiding in rapid decision-making. They are particularly useful in scenarios where traditional skin tests fall short, such as in populations previously vaccinated with BCG. In public health initiatives, the factory-produced tests facilitate early diagnosis and contribute significantly to TB control efforts, especially in communities with limited access to comprehensive healthcare facilities.
Product After-Sales Service
QL Biotech provides comprehensive after-sales support, including detailed product manuals, troubleshooting guidance, and customer service contacts for any inquiries regarding the Tuberculosis Blood Test device. Our factory ensures that replacement parts and additional supplies are readily available for efficient operation in clinical settings.
Product Transportation
Our Tuberculosis Blood Test devices are packaged to withstand transportation, ensuring safe delivery to various healthcare facilities worldwide. We adhere to international shipping standards to maintain product integrity.
Product Advantages
The test offers high specificity and sensitivity, quick results within 15 minutes, and eliminates the need for a return visit, making it an efficient choice for TB screening in various healthcare scenarios.
Product FAQ
- Q: How reliable is the Tuberculosis Blood Test?
A: The factory-produced IGRA test offers high reliability due to its specificity and sensitivity, reducing false positives associated with skin tests, particularly in BCG vaccinated individuals. - Q: How is the test administered?
A: The test requires a sample of whole blood, serum, or plasma. The specimen is mixed with sample diluent and added to the test device, with results ready in 15 minutes. - Q: Can the test be used in all populations?
A: While highly effective, the IGRA may show reduced sensitivity in certain groups such as young children or immunocompromised individuals, a common consideration across TB tests. - Q: Is special equipment necessary to perform the test?
A: No special equipment is needed. The test is designed for ease of use in various clinical settings, making it highly accessible. - Q: What is the shelf life of the test device?
A: The device has a shelf life of up to 2 years when stored under proper conditions (2-30°C), ensuring long-term usability. - Q: Does the test distinguish between active and latent TB?
A: The test indicates TB infection but cannot differentiate between active and latent TB; clinical evaluation is necessary for further diagnosis. - Q: How quick are the results available?
A: Results are available within 15 minutes, allowing for rapid clinical decision-making and efficient patient management. - Q: Are there any contraindications for this test?
A: There are no significant contraindications, but the test should be performed by trained personnel to ensure accurate results. - Q: What should be done if the test is positive?
A: A positive result should be followed by further diagnostic evaluation, including medical history and additional testing to confirm TB status. - Q: Can this test be used for routine screenings?
A: Yes, the test is suitable for routine TB screenings, particularly in high-risk or high-prevalence environments, thanks to its quick and reliable results.
Product Hot Topics
- The Role of Factory-Made Tuberculosis Blood Tests in Public Health
Factory-manufactured Tuberculosis Blood Tests have become essential tools in public health strategies, providing an efficient means of TB screening. These tests are crucial in areas with prevalent TB cases and limited healthcare resources. By facilitating early and accurate detection, they help in controlling the spread of TB, allowing health authorities to implement timely interventions. The reliability and quick turnaround of results make these factory-produced tests a preferred choice in both developed and developing regions, supporting global health initiatives aimed at eradicating TB. - Innovations in Factory-Based Production of TB Diagnostics
The advancement in factory-based production of Tuberculosis Blood Tests has revolutionized TB diagnostics. Factories like QL Biotech have optimized manufacturing processes to enhance the accuracy and efficiency of TB tests. By utilizing state-of-the-art technology and rigorous quality control, these factories ensure that the TB Blood Tests meet the highest standards. This innovation not only boosts the diagnostic accuracy but also ensures large-scale availability, addressing the need for robust diagnostic tools in the fight against TB globally.
Image Description