Fast & Reliable Hbsag Test Cassette for H. Pylori Detection
PRINCIPLE
The H. Pylori Ab Rapid Test Strip (Serum/ Plasma) is a qualitative membrane strip based immunoassay for the detection of H. Pylori antibodies in whole blood, serum or plasma. In this test procedure, the test strip is immersed in the specimen or specimen containing buffering solution. This specimen migrates chromatographically along the length of the test strip and interacts with the reagents contained on the test strip. If the specimen contains H. Pylori antibodies, a colored line will appear in the test line region indicating a positive result. If the specimen does not contain H.
Pylori antibodies, a colored line will not appear in this region indicating a negative result. To serve as a procedural control, a colored line will always appear at the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.
Product Detail
Brand: QL
Specimens: : Serum/Plasma
Reading time:20 minutes.
Pack:25 T
STORAGE: 2‐30°C
KIT COMPONENTS(Device)
Individually packed test devices/strips
Disposable pipettes
Buffer
Package insert
Brand: QL
Specimens: : Whole Blood/Serum/Plasma
Reading time:20 minutes.
Pack:25 T
STORAGE: 2‐30°C
Individually packed test devices/strips
Disposable pipettes
Buffer
Package insert
PROCEDURE
- Allow the test device, specimen, buffer, and/or controls to equilibrate to room temperature (15-30°C) prior to testing.
- Remove the test device from the foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
- Place the test device on a clean and level surface.
- Hold the dropper vertically and transfer 2 drops of sample to the specimen well (S) of the test device, then add 1 drop of buffer and start the timer.
- Wait for the red line(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 20 minutes.
INTERPRETATION OF RESULTS
-
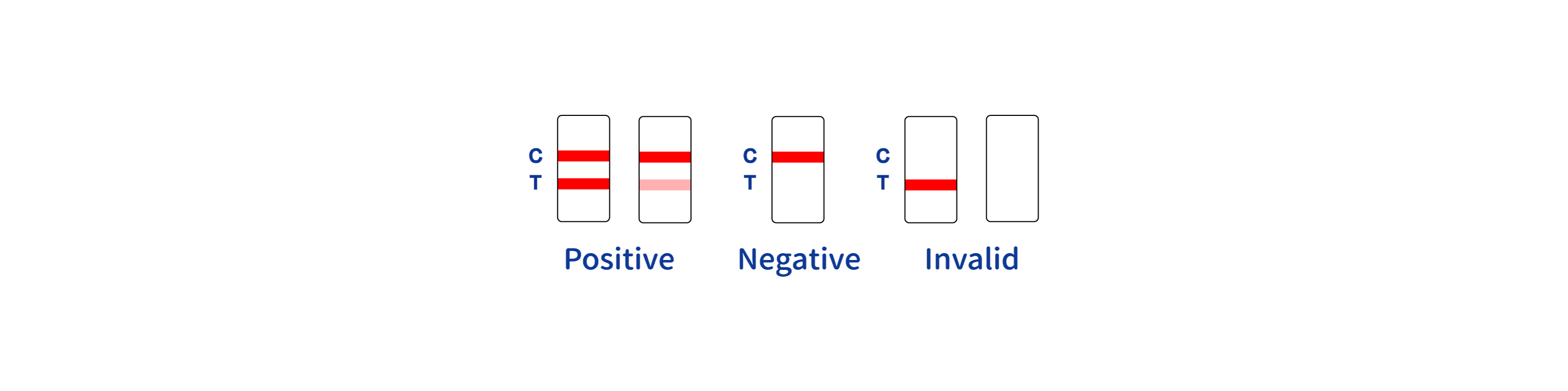
POSITIVE RESULT:
* A colored band appears in the control band region (C) and another colored band appears in the T band region.
NEGATIVE RESULT:
One colored band appears in the control band region (C). No band appears in the test band region (T).
INVALID RESULT:
Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor.
NOTE:
1. The intensity of the color in test region (T) may vary depending on the concentration of aimed substances present in the specimen. Therefore, any shade of color in the test region should be considered positive. Besides, the substances level can not be determined by this qualitative test.
2. Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
Upon delving deeper into the operational facets of the Hbsag Test Cassette, users will find an unparalleled ease of application. The process begins with the collection of a minimal sample volume, which is then applied to the specimen pad of the test strip. As the sample migrates through the device, it encounters a conjugate pad containing antibodies specific to the H. Pylori antigen, conjugated with colloidal gold particles. In the presence of H. Pylori antibodies, a complex forms, which is then captured on a test line in the result window, revealing a colored line indicative of a positive outcome. This simplistic yet thorough approach not only ensures high specificity and sensitivity but also delivers results within a remarkably short time frame—a paramount feature in fast-paced clinical settings or for point-of-care testing where every second counts. QL Biotech's commitment to advancing healthcare diagnostics propels the Hbsag Test Cassette into the forefront of medical innovation. With every aspect meticulously crafted, from the intuitive design to the comprehensive instruction manual, this product ensures a seamless user experience. Whether employed in hospitals, clinics, or for in-home testing, the Hbsag Test Cassette signifies a stride towards more accessible, efficient, and accurate healthcare solutions, embodying QL Biotech's mission to empower professionals and individuals alike in the battle against H. Pylori infections.


