Fast & Reliable Syphilis Test at Home Kit - QL Biotech
PRINCIPLE
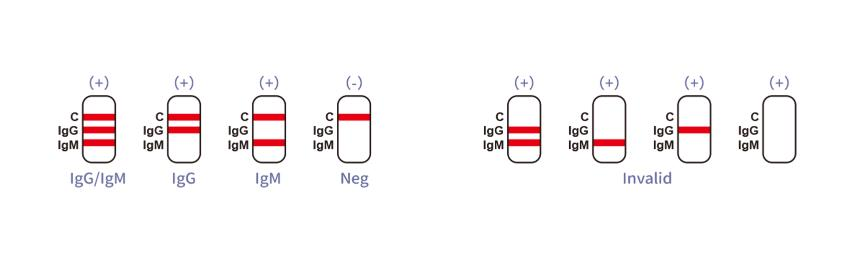
The Typhoid IgG/IgM Rapid Test Device is a lateral flow chromatographic immunoassay. The test cassette consists of: 1) a burgundy colored conjugate pad containing recombinant S. typhoid H antigen and O antigen conjugated with colloid gold (Typhoid conjugates) and rabbit IgG-gold conjugates, 2) a nitrocellulose membrane strip containing two test bands (IgG and IgM bands) and a control band (C band). The IgM band is pre-coated with monoclonal anti-human IgM for the detection of IgM anti-S. typhi, IgG band is pre-coated with reagents for the detection of IgG anti-S. typhi , and the C band is pre-coated with goat anti rabbit IgG.
When an adequate volume of test specimen is dispensed into the sample well of the cassette, the test specimen migrates by capillary action across the test cassette. Anti-S. typhi IgM if present in the patient specimen will bind to the Typhoid conjugates. The immunocomplex is then captured on the membrane by the pre-coated anti-human IgM antibody, forming a burgundy colored IgM band, indicating a S. typhi IgM positive test result.
Anti-S. typhi IgG if present in the patient specimen will bind to the Typhoid conjugates. The immunocomplex is then captured by the pre-coated reagents on the membrane, forming a burgundy colored IgG band, indicating a S. typhi IgG positive test result.
Absence of any test bands suggests a negative result. The test contains an internal control (C band) which should exhibit a burgundy colored band of the immunocomplex of goat anti rabbit
IgG/rabbit IgG-gold conjugate regardless of the color development on any of the test bands.
Otherwise, the test result is invalid and the specimen must be retested with another device.
Product Detail
-
-
-
-
-
-
-
1.Brand: QL
Specimens: :Whole Blood/Serum/Plasma
Reading time:10 minutes.
Pack:25 T
STORAGE: 2‐30°C
KIT COMPONENTS
- Test devices
- Droppers
- Buffer
- Package insert
2.Brand: QL
Specimens: :Serum/Plasma
Reading time:1 minutes.
Pack:25 T
STORAGE: 2‐30°C
KIT COMPONENTS
- Test devices
- Droppers
- Buffer
- Package insert
-
-
-
-
-
-
-
ASSAY PROCEDURE
-
1.Typhoid IgG/IgM Rapid Test Device(Whole Blood/Serum/Plasma)Allow test device, specimen, buffer and/or controls to reach room temperature (15-30°C) prior to testing.
1. Bring the pouch to room temperature before opening it. Remove the test device from the sealed pouch and use it as soon as possible.
2. Place the test device on a clean and level surface.
For Whole Blood, Serum or Plasma specimens:
Hold the dropper vertically and transfer 2 drops of specimen (or approximately 50 µL) to the specimen well (S) of the test device, then add 1 drop of buffer and start the timer.
For Fingerstick Whole Blood specimens:
To use a capillary tube: Fill the capillary tube and transfer approximately 50 µL (or 2 drops) of fingerstick whole blood specimen to the specimen well (S) of the test device, then add 1 drop of buffer and start the timer.
3. Wait for the colored line(s) to appear. Read results at 10 minutes. Do not interpret results after 20 minutes.
2 Typhoid IgG/IgM Rapid Test Device(Serum/Plasma)
1. Bring the specimen and test components to room temperature. Open the pouch at the notch and remove device. Place the test device on a clean, flat surface.
2. Fill the pipette dropper with the specimen. Holding the dropper vertically, dispense 1 drop (about 40 µL) of specimen into the sample well making sure that there are no air bubbles. Then add 1 drop (about 35-50 µL) of Sample Diluent immediately. Set up timer.
3. Results can be read in 15 minutes. Positive results can be visible in as short as 1 minute. Don’t read result after 15 minutes.
INTERPRETATION OF ASSAY RESULT
Typhoid IgG/IgM
-
NOTE:
-
- 1.The intensity of the color in test region (T) may vary depending on the concentration of aimed substances present in the specimen. Therefore, any shade of color in the test region should be considered positive. Besides, the substances level cannot be determined by this qualitative test.
- 2.Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
Understanding the principles behind the Typhoid IgG/IgM Rapid Test Device provides insight into its adeptness at syphilis detection. The device operates on a lateral flow chromatographic immunoassay method, now ingeniously adapted for home syphilis testing. This allows individuals to conduct the test with ease, offering results that are both quick and accurate. The beauty of this device lies in its simplicity - a sophisticated diagnostic tool that does not compromise on performance or reliability when repurposed for Syphilis screening. It ensures that anyone can perform a Syphilis Test at Home without the need for specialized training or equipment, bridging the gap between professional healthcare diagnostics and home use. QL Biotech is proud to bring this innovative solution into the realm of personal health management. The adaptation of our proven Typhoid IgG/IgM Rapid Test Device for syphilis testing underscores our commitment to empowering individuals with the tools needed for proactive health monitoring. In doing so, we are expanding the possibilities for discreet, home-based testing, making it easier than ever for people to take control of their sexual health. Our device not only marks a significant advancement in at-home diagnostic testing but also reflects QL Biotech’s ongoing dedication to innovation, accuracy, and accessibility in healthcare.


