HIV AG Rapid Test - Quick Detection for Early Diagnosis
PRINCIPLE
Human Immunodeficiency Virus (HIV) Ab & P24 Ag Rapid Test Device (Whole Blood/Serum/Plasma) is a qualitative, membrane based immunoassay for the detection of antibodies to HIV 1&2 and P24 antigen in whole blood, serum or plasma. The membrane is precoated with recombinant HIV antigens to HIV 1&2 antibody and P24 antibody to HIV P24 antigen. During testing, the whole blood, serum or plasma specimen reacts with HIV antigen or P24 antibody coated particles in the test strip. The mixture then migrates upward on the membrane chromatographically by capillary action and reacts with recombinant HIV antigen or P24 antibody on the membrane in the test line regions. If the specimen contains antibodies to HIV 1 and/or HIV 2 and/or P24 antigen, a colored line will appear in the test line region indicating a positive result. If the specimen does not contain HIV 1 and/or HIV 2 antibodies and/or P24 antigen, no colored line will appear in the test line region indicating a negative result. To serve as a procedural control, a colored line will always appear in the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.
MATERIALS
Materials Provided
● Test devices ● Disposable specimen droppers
● Buffer ● Package insert
Materials Required But Not Provided
● Specimen collection containers ● Lancets (for fingerstick whole blood only)
● Centrifuge (for plasma only) ● Timer
● Disposable heparinized capillary tubes and dispensing bulb (for fingerstick whole blood only)
DIRECTIONS FOR USE
-
● Test devices
● Disposable specimen droppers
● Buffer
● Package insert
Materials Required But Not Provided
● Specimen collection containers
● Lancets (for fingerstick whole blood only)
● Centrifuge (for plasma only)
● Timer
● Disposable heparinized capillary tubes and dispensing bulb (for fingerstick whole blood only)
PERFORMANCE CHARACTERISTICS
Sensitivity
Human Immunodeficiency Virus (HIV) Ab & P24 Ag Rapid Test Device (Whole
Blood/Serum/Plasma) has been tested by anti-HIV 1 low titer performance panel, anti-HIV 2 performance panel and anti-HIV 1 seroconversion panel (Boston Biomedica, Inc.). And it has also been compared with leading commercial EIA HIV test on clinical specimens. The results show that
HIV 1&2 Human Immunodeficiency Virus Rapid Test Device (Whole Blood/Serum/Plasma) is very sensitive to HIV 1 and/or HIV 2 antibodies.
Specificity
The specificity of the test is comparable to a leading commercial HIV EIA test. Human Immunodeficiency Virus (HIV) Ab & P24 Ag Rapid Test Device (Whole Blood/Serum/Plasma) is highly specific for anti-HIV 1 and/or HIV 2 compared to a leading commercial HIV EIA test.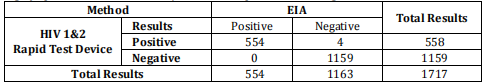
Relative Sensitivity: 99.9% (99.3%-100.0%)*Relative Specificity: 99.6% (99.1%-99.9%)*
Relative Accuracy: 99.8% (99.4%-99.9%)* * 95% Confidence Interval
Precision
Intra Assay
Within-run precision has been determined by using 15 replicates of three specimens: a negative, a low positive and a high positive. The negative, low positive and high positive values were correctly identified 99.5% of the time.
Inter-Assay
Between-run precision has been determined by 15 independent assays on the same three specimens: a negative, a low positive and a high positive. Three different lots of Human
Immunodeficiency Virus (HIV) Ab & P24 Ag Rapid Test Device (Whole Blood/Serum/Plasma) have been tested using negative, low positive and high positive specimens. The specimens were correctly identified 99.5% of the time.
Our test leverages a sophisticated, membrane-based immunoassay technology to qualitatively detect the presence of antibodies to HIV types 1 and 2, as well as the P24 antigen, within a remarkably short time frame. This dual detection capability enhances the test's sensitivity and specificity, making it an invaluable asset in the early stages of HIV infection when P24 antigen levels are particularly high even before antibodies are fully detectable. The simple, user-friendly design of our test kit ensures that it can be administered with ease in a variety of settings, from advanced diagnostic labs to remote field locations, providing critical support in global efforts to curb the spread of HIV. With the HIV AG Rapid Test Device by QL Biotech, early detection is now more accessible than ever, empowering healthcare professionals and patients alike with the knowledge needed to take timely action.


