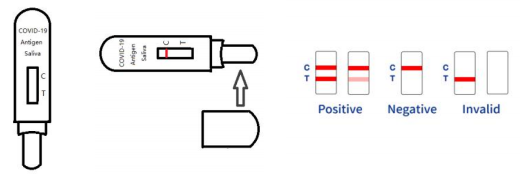
Influenza A/B Rapid Test Kit Manufacturer QL Biotech
Product Main Parameters
| Parameter | Value |
|---|---|
| Test Type | Rapid Visual Immunoassay |
| Sample Type | Nasal/Nasopharyngeal swabs |
| Detection Time | 15-20 minutes |
| Sensitivity | 95% |
| Specificity | 99% |
Common Product Specifications
| Specification | Details |
|---|---|
| Kit Contents | Test device, buffer, swabs, instructions |
| Shelf Life | 12 months |
| Storage Conditions | 2-30°C |
Product Manufacturing Process
The manufacturing process of Influenza A/B rapid test kits involves several critical steps ensuring accuracy and reliability. Initially, high-quality monoclonal antibodies specific for Influenza A and B are developed. These antibodies are then conjugated with colloidal gold particles to serve as indicators in the test strip. The lateral flow test strips are assembled by coating capture antibodies on nitrocellulose membranes, ensuring proper alignment and sensitivity. The assembled strips undergo rigorous quality control tests to confirm performance standards. A study from the Journal of Clinical Virology explores such processes, highlighting the importance of antibody selection and quality control in enhancing test accuracy.
Product Application Scenarios
Influenza A/B rapid test kits are vital tools in various public health settings. They are extensively used for early detection of flu viruses in clinics, hospitals, and laboratories. Rapid testing allows healthcare professionals to quickly identify and isolate flu cases, minimizing transmission. A study in The Lancet emphasizes the role of rapid diagnostic kits in controlling outbreaks, reducing the burden on healthcare facilities, and guiding appropriate antiviral treatments. Moreover, their application extends to community healthcare services, providing an efficient tool for mass screenings during flu seasons.
Product After-Sales Service
QL Biotech offers comprehensive after-sales service to ensure customer satisfaction and product reliability. This includes technical support, product training, and replacement services for defective kits. Customers can reach out via hotline or email for any inquiries or issues. Our expert team is dedicated to resolving all concerns promptly, ensuring continued trust and cooperation.
Product Transportation
Our Influenza A/B rapid test kits are meticulously packaged to withstand transportation challenges. We use climate-controlled logistics to maintain the integrity of the products, ensuring they arrive in optimal condition. Orders are tracked throughout the delivery process, and any issues are promptly addressed.
Product Advantages
- High accuracy with a sensitivity of 95% and specificity of 99%.
- Rapid results within 15-20 minutes.
- Easy to use in various settings.
- Comprehensive technical support.
- Manufactured by a trusted company with over 20 years of experience.
Product FAQ
- Q: What sample types can be used with this test?
The Influenza A/B rapid test is compatible with both nasal and nasopharyngeal swabs, ensuring versatility in sample collection.
- Q: How should the test be stored?
Tests should be stored at temperatures between 2-30°C, away from direct sunlight and moisture, to maintain efficacy during their 12-month shelf life.
- Q: Can the test differentiate between Influenza A and B?
Yes, the test can distinguish between Influenza A and B, providing specific results for each virus type.
- Q: Is the test suitable for home use?
While intended for professional use, the test can be administered in home settings by trained individuals following the provided instructions.
- Q: What is the required detection time?
The test delivers results within 15-20 minutes, making it ideal for rapid screening needs.
- Q: How reliable are the results?
With high specificity and sensitivity, our test is highly reliable for detecting Influenza A and B, ensuring accurate diagnosis.
- Q: Are there any known cross-reactions?
The test is designed to minimize cross-reactivity, ensuring accuracy. Any such concerns are addressed during quality control.
- Q: What should be done if a test result is invalid?
An invalid result typically arises from user error. Retesting with a new kit is recommended, and following the instructions carefully should help avoid this.
- Q: How does this test compare to PCR testing?
While PCR is a gold standard for diagnosis, rapid tests like ours provide quicker results with reasonable accuracy, aiding immediate decisions.
- Q: Is training available for administering the test?
QL Biotech provides comprehensive training materials and support to ensure the correct administration of the test for accurate results.
Product Hot Topics
- Comment: The Evolution of Rapid Testing in Influenza Detection
As healthcare challenges continue to evolve, rapid testing remains at the forefront for addressing respiratory diseases like influenza. In recent years, rapid diagnostic tests, such as those for Influenza A/B, have become invaluable tools in clinical settings. They offer a quick turnaround time, which is crucial in managing influenza outbreaks. The predictability and reliability of these tests allow healthcare providers to implement timely interventions, ultimately reducing transmission rates. Manufacturers like QL Biotech have continued to improve the accuracy and ease of use of their rapid tests, ensuring they stay ahead of the curve. The commitment to innovation ensures these tests remain a staple in fighting influenza seasons, offering peace of mind to patients and practitioners alike.
- Comment: Influenza A/B Testing in Pandemic Preparedness
The rapid spread of infectious diseases has underscored the importance of pandemic preparedness, with rapid testing playing a critical role. Influenza A/B tests stand out due to their ability to provide fast and reliable diagnostics, essential for curbing outbreaks. QL Biotech, as a manufacturer, ensures these kits are designed for precision and ease of use, making them suitable for widespread use in various healthcare settings. The role of these tests extends beyond mere diagnosis; they are pivotal in guiding public health decisions, such as vaccination strategies and antiviral deployments. As we face ever-evolving viral threats, the importance of rapid test manufacturers in global health security cannot be overstated.
Image Description