Innovative CMC Combo Test: Troponin T Rapid Analysis Device
MATERIALS

PRINCIPLE
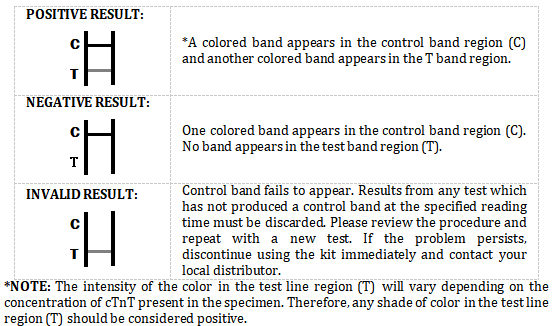
The Troponin T Rapid Test Device (Whole Blood/Serum/Plasma) is a qualitative, membrane based immunoassay for the detection of cTnT in whole blood, serum or plasma. The membrane is pre-coated with Strepavidin-IgG on the test line region of the test and cTnT-biotin on the sample pad. During testing, the whole blood, serum or plasma specimen reacts with the cTnT-biotin on the sample pad frist, then migrates and react with the particle coated with anti-cTnT antibodies. The mixture migrates upward on the membrane chromatographically by capillary action to react with capture reagent on the membrane and generate a colored line. The presence of this colored line in the test line region indicates a positive result, while its absence indicates a negative result. To serve as a procedural control, a colored line will always appear in the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.
INTERPRETATION OF RESULTS
PERFORMANCE CHARACTERISTICS
Sensitivity and Specificity
The Troponin T Rapid Test Device (Whole Blood/Serum/Plasma) has been evaluated with a leading commercial cTnT EIA test using clinical specimens. The results show that the sensitivity of the Troponin T Rapid Test Device (Whole Blood/Serum/Plasma) is 98.3% and the specificity is 98.4% relative to the leading EIA test.
Troponin T Rapid Test Device vs. EIA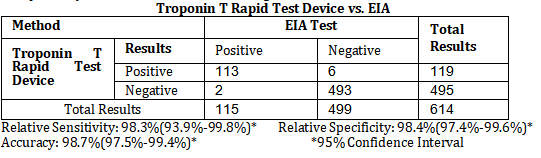
Please note: Use as directed by a medical professional. Read all instructions thoroughly before use. Always use appropriate safety measures when handling blood, serum, or plasma samples. QL Biotech - advancing the field of rapid medical diagnostics one test at a time.


