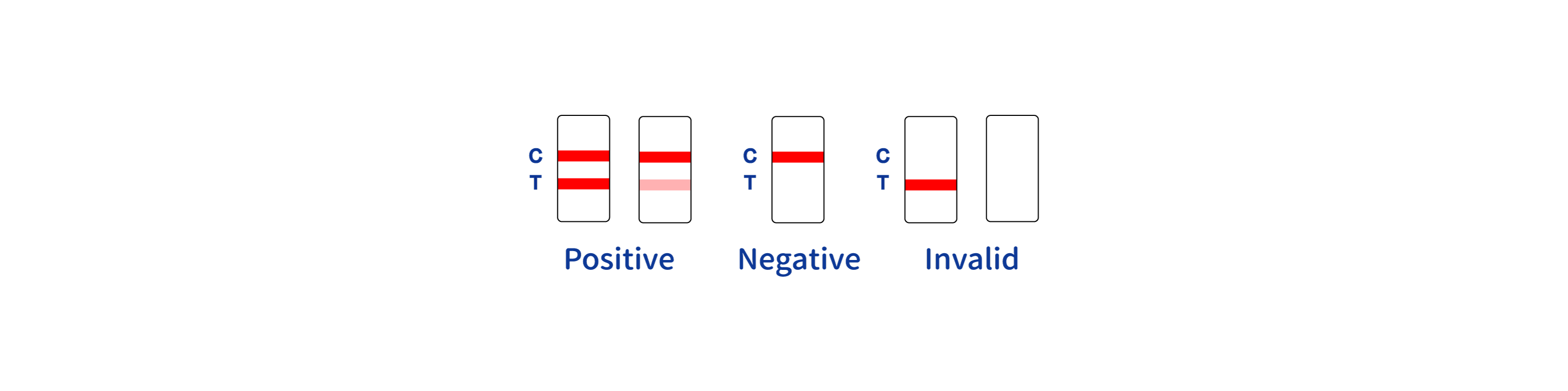
Manufacturer Blood Dengue Test Kit - Rapid Detection
Blood Dengue Test Kit Product Details
| Parameter | Details |
|---|---|
| Test Type | Molecular and Serological Tests |
| Reading Time | 10 minutes |
| Storage Temperature | 2-30°C |
| Pack Size | 20 T |
Common Product Specifications
| Specification | Description |
|---|---|
| Specimen Type | Blood |
| Test Method | RT-PCR, NS1 Antigen, IgM, IgG |
| Sensitivity | High |
| Specificity | High |
Product Manufacturing Process
The manufacturing process of the Blood Dengue Test Kit involves several critical steps to ensure accuracy and reliability. Initially, raw materials are carefully selected and tested for quality assurance. The RT-PCR test component involves sophisticated techniques where viral RNA is extracted from blood samples, followed by its amplification to detect the presence of the dengue virus. The serological tests for NS1 antigen and antibodies (IgM and IgG) involve the preparation of conjugates, which are then applied to test strips. Each strip undergoes rigorous testing through simulated assays to guarantee performance standards are met. Final products are packaged under sterile conditions, adhering to international regulatory standards for diagnostic devices. The manufacturer continually invests in research and development to enhance the kit’s efficacy, confirming its position as a reliable solution in the IVD market.
Product Application Scenarios
The Blood Dengue Test Kit by the manufacturer is versatile in its application, ideal for use in hospitals, clinics, and remote health centers. Its rapid diagnostic capability is crucial for tropical and subtropical regions prone to dengue outbreaks. By identifying the virus early, healthcare professionals can implement timely interventions, reducing disease severity and preventing complications. The kit is also instrumental in epidemiological surveillance, assisting public health authorities to monitor and respond to outbreaks effectively. In clinic settings, its ease of use and minimal requirement for advanced equipment make it an accessible choice for efficient patient management. Overall, the kit’s application extends beyond diagnosis, aiding in comprehensive public health strategies to control dengue transmission.
Product After-Sales Service
The manufacturer offers comprehensive after-sales service, including user training, technical support, and a warranty for defective kits. Customers can access online resources and contact service centers for assistance.
Product Transportation
Blood Dengue Test Kits are meticulously packaged to maintain product integrity during transit. The manufacturer partners with reliable logistics services to ensure timely and safe delivery worldwide.
Product Advantages
- Rapid and reliable diagnosis for efficient disease management.
- High specificity and sensitivity enhance diagnostic confidence.
- Ease of use in various healthcare settings, including limited-resource areas.
- Supports public health initiatives through effective surveillance.
Product FAQ
- What is the principle behind the Blood Dengue Test Kit?
The kit uses molecular and serological methods to detect dengue virus or related antibodies in blood samples, providing rapid results. - How accurate is the Blood Dengue Test Kit from this manufacturer?
The test kit is designed with high sensitivity and specificity, ensuring reliable and accurate results when used correctly. - Can the kit be used in remote or low-resource settings?
Yes, the test kit's design allows for use with minimal laboratory infrastructure, making it suitable for remote locations. - What kind of samples are required for the test?
The Blood Dengue Test Kit requires blood samples to perform the diagnostic procedures accurately. - What is the storage condition for the test kit?
The test kit should be stored at temperatures between 2-30°C to maintain its efficacy. - How soon can the results be read after performing the test?
Results can typically be read within 10 minutes after the test is initiated. - Is there any cross-reactivity with other viruses?
While designed for specificity, some serological tests may exhibit cross-reactivity with other flaviviruses like Zika; molecular tests are more precise. - What training is required to use the test kit?
Basic laboratory training is recommended to ensure proper handling and interpretation of the test results. - Are there any guidelines for interpreting the results?
Yes, the manufacturer provides comprehensive instructions and guidelines with each kit for result interpretation. - What support is available post-purchase?
The manufacturer provides technical support, training resources, and a warranty service to address any customer concerns.
Product Hot Topics
- How does the Blood Dengue Test Kit contribute to global public health?
The kit plays a critical role in countries facing frequent dengue outbreaks, providing essential diagnostic capabilities that enable timely medical interventions. Its implementation aids in controlling spread, supports efficient resource allocation, and guides public health responses. The manufacturer’s test kits also assist in research and surveillance, contributing valuable data for epidemiological studies that inform future health policies and strategies globally. - The impact of early diagnosis using the Blood Dengue Test Kit
Early diagnosis of dengue infection is vital in reducing mortality rates and managing the disease effectively. The manufacturer’s Blood Dengue Test Kit offers rapid diagnosis capabilities, helping healthcare providers administer timely treatments that can prevent severe symptoms. By identifying the virus early, healthcare systems can also manage patient flow better during peak dengue seasons, maximizing the use of available medical resources. - The evolution of diagnostic technology in Blood Dengue Test Kits
Over the years, diagnostic technology for dengue has evolved significantly, with manufacturers like this one leading the charge. The incorporation of molecular techniques such as RT-PCR has refined the detection process, offering unprecedented accuracy. Continued innovation focuses on making these technologies more accessible, cost-effective, and adaptable to different healthcare settings, reinforcing the role of diagnostic tools in combating infectious diseases. - Addressing the challenges of dengue diagnosis in low-resource areas
In regions with limited healthcare infrastructure, accessing reliable diagnostics can be challenging. The manufacturer’s Blood Dengue Test Kit addresses these issues through its user-friendly design that requires minimal equipment. This adaptability ensures that remote areas can also benefit from accurate dengue detection, bridging the gap in healthcare delivery and aiding in effective disease management even in isolated communities. - Prospects of Blood Dengue Test Kits in epidemic forecasting
The reliable data garnered from widespread use of Blood Dengue Test Kits can empower predictive modeling, aiding in epidemic forecasting. This manufacturer’s test kits, through rapid deployment and ease of use, provide timely insights into disease spread patterns. Such data is invaluable for preemptive public health actions, potentially averting large-scale outbreaks by informing strategic interventions well in advance. - Understanding the science behind dengue serological tests
Dengue serological tests, integral to the manufacturer’s Blood Dengue Test Kit, detect antibodies that signify recent or past infections. These tests, including NS1 antigen detection, offer insights into the body’s immune response to dengue virus. By understanding these mechanisms, healthcare providers can better interpret diagnostic results and tailor treatment plans according to the infection stage, enhancing patient outcomes. - Exploring the role of RT-PCR in dengue virus detection
RT-PCR technology remains a cornerstone in the manufacturer’s Blood Dengue Test Kit for its accuracy in detecting viral RNA in the early stages of infection. This method amplifies the virus's genetic material, allowing for precise detection even at low concentrations. Its role in confirming dengue infection early helps mitigate the disease’s impact, affording patients a greater chance of rapid recovery. - Challenges and opportunities in manufacturing diagnostic kits
The process of manufacturing diagnostic kits, such as the Blood Dengue Test Kit, involves navigating complex challenges, including ensuring product quality, regulatory compliance, and cost efficiency. Manufacturers must balance these aspects while innovating to meet evolving healthcare needs. This sector, however, offers significant opportunities for growth and impact, particularly as global health systems prioritize early and accurate disease detection. - The importance of after-sales service in IVD products
In the IVD sector, effective after-sales service enhances user experience and product trust. The manufacturer’s commitment to providing comprehensive support, including user training and technical assistance for their Blood Dengue Test Kit, ensures that customers can maximize the product’s potential. This support system is crucial in maintaining customer satisfaction and loyalty, reinforcing the manufacturer’s reputation in the healthcare industry. - Innovations driving the future of dengue diagnostics
The future of dengue diagnostics is poised for transformation, with innovations focusing on improving accessibility and accuracy. The manufacturer’s efforts in advancing their Blood Dengue Test Kit exemplify this trend, with ongoing research towards portable and cost-effective solutions. These innovations are expected to play a pivotal role in enhancing global health outcomes by empowering more communities with reliable diagnostic tools.
Image Description