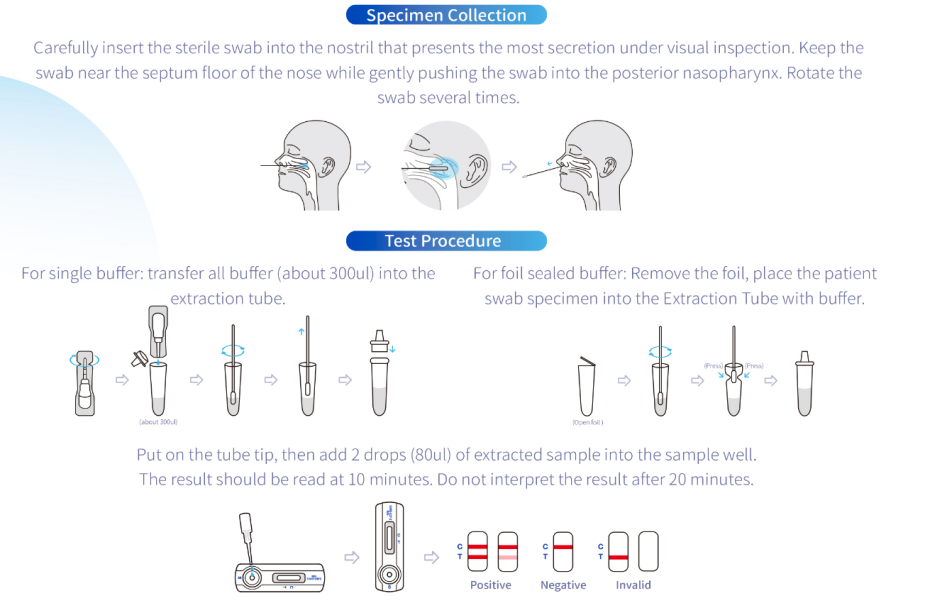
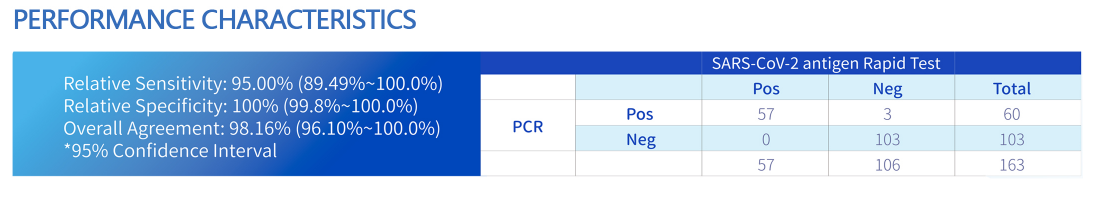
Manufacturer Flu Antigen Test by QL Biotech
Product Main Parameters
| Specifications | Details |
|---|---|
| Brand | QL Biotech |
| Certificate | CE, ISO13485 |
| Specimen | Nasopharyngeal/Nasal swabs |
| Reading Time | 10 minutes |
| Pack | 20 Tests |
| Shelf Life | 2 Years |
Common Product Specifications
| Storage Temperature | 2-30°C |
| Storage Conditions | Sealed pouch, no freezing |
Product Manufacturing Process
The manufacturing process of the Flu Antigen Test by QL Biotech follows strict adherence to quality guidelines. As described in authoritative papers, the process begins with the selection of high-quality raw materials, followed by formulation under sterile conditions to prevent contamination. The test components are then assembled, with reagents being carefully measured to ensure accuracy. Quality control tests are conducted at various stages to maintain high sensitivity and specificity. Finally, the tests are packaged with detailed instructions to maintain integrity during transport and storage. QL Biotech, the manufacturer, ensures that each step complies with international standards, providing healthcare providers with reliable diagnostic tools.
Product Application Scenarios
Flu Antigen Test by QL Biotech is crucial in various clinical scenarios. Authoritative literature emphasizes its use in outpatient settings like clinics and emergency rooms, where rapid diagnosis is critical. The test aids in distinguishing influenza from other respiratory infections, facilitating timely antiviral treatments. During flu season, rapid and accurate detection helps manage patient care and prevent widespread outbreaks. QL Biotech's Flu Antigen Test supports healthcare professionals in making informed decisions, optimizing patient outcomes, and reducing the flu burden on healthcare systems.
Product After-Sales Service
QL Biotech, the manufacturer, offers comprehensive after-sales support for the Flu Antigen Test. This includes customer service for troubleshooting, guidance on test usage, and warranty for manufacturing defects. A dedicated helpline and email support ensure timely responses to any technical issues.
Product Transportation
The Flu Antigen Test by QL Biotech is packaged securely to prevent damage during transportation. The kits are stored in a controlled environment, maintaining the recommended temperature and humidity levels to preserve product efficacy. Detailed shipping information accompanies each order, ensuring safe delivery.
Product Advantages
- Rapid results within 10 minutes for timely patient management.
- High specificity to accurately detect influenza antigens.
- Convenient packaging for ease of use in various clinical settings.
- Manufactured by QL Biotech, ensuring adherence to international standards.
- Cost-effective solution for influenza diagnostics.
Product FAQ
- How accurate is the Flu Antigen Test by the manufacturer?
The Flu Antigen Test by QL Biotech has a high specificity, ensuring accurate detection of influenza antigens. However, like all RIDTs, sensitivity may vary, and false negatives can occur, especially with low viral loads. It is essential to consider additional clinical evaluations for comprehensive diagnosis.
- Can the test be used for all ages?
Yes, the test can be utilized across various age groups. However, appropriate sample collection techniques should be employed to ensure optimal results. The manufacturer's instructions provide guidance for obtaining accurate specimens from patients of all ages.
- What should be done in case of a negative result but high clinical suspicion?
If a negative result is obtained but clinical suspicion for influenza remains high, healthcare providers should consider using more sensitive assays like PCR tests for confirmation. Clinical judgment, along with additional testing, helps ensure accurate diagnosis.
- Is it necessary to use a particular swab brand for this test?
While it is recommended to use the sterile swabs provided in the kit, healthcare providers can use standard nasopharyngeal or nasal swabs if necessary. However, it is crucial to ensure that swabs meet clinical standards to avoid compromising test accuracy.
- How does temperature affect the test's performance?
The Flu Antigen Test should be stored and used within the recommended temperature range of 2-30°C. Extreme temperatures can affect reagents and overall test efficacy, leading to inaccurate results. Transport and storage should adhere to these guidelines.
- How does the manufacturer ensure quality control?
QL Biotech implements stringent quality control measures at each step of the manufacturing process. Routine testing for sensitivity, specificity, and sterility ensures that only high-quality products reach healthcare providers. Certificates CE and ISO13485 guarantee conformity with international standards.
- What is the shelf life of the Flu Antigen Test?
The Flu Antigen Test by QL Biotech has a shelf life of 2 years from the date of manufacture. Proper storage conditions, as outlined by the manufacturer, help maintain the test's efficacy throughout this period.
- Is the test affected by cross-reactivity with other viruses?
QL Biotech's Flu Antigen Test is designed to target specific influenza antigens, minimizing cross-reactivity. However, like all immunoassays, some potential for cross-reactivity exists, emphasizing the need for correlation with clinical findings.
- What formats are available for the test?
The test is available in cassette format, with each package containing 20 tests. This format simplifies testing procedures and ensures easy application in clinical settings.
- What is included in the test kit?
Each kit includes 20 test cassettes, sterile swabs, single extraction buffers, a package insert, and a quick reference guide. This comprehensive set allows healthcare providers to conduct the test efficiently.
Product Hot Topics
- Advancements in Flu Antigen Testing by Manufacturer
Recent studies published in authoritative journals highlight the ongoing advancements in rapid diagnostic testing led by manufacturers like QL Biotech. With an emphasis on improving sensitivity and specificity, newer tests are showing promise in providing even more reliable influenza diagnostics. As we navigate through seasonal outbreaks and unexpected viral challenges, these innovations become pivotal in healthcare decision-making. The focus is not only on influenza but also on adaptable platforms that can detect multiple pathogens, demonstrating the manufacturer's commitment to evolving healthcare needs.
- The Role of Flu Antigen Tests in Pandemic Preparedness
Flu Antigen Tests, such as those manufactured by QL Biotech, play a crucial role in pandemic preparedness plans. Their rapid turnaround time and ease of use in various healthcare settings make them valuable tools in quickly identifying and managing influenza outbreaks. As global health policies prioritize early detection and intervention, manufacturers are responding with enhanced test designs that promise greater accuracy and operational flexibility. The strategic deployment of these tests can significantly mitigate the impact of widespread respiratory infections, ensuring public health safety.
- Comparing Flu Antigen Test Manufacturers: What Sets QL Biotech Apart?
When evaluating flu antigen test manufacturers, factors such as quality control, product innovation, and customer support become pivotal. QL Biotech distinguishes itself through its rigorous adherence to international standards, including CE and ISO13485 certifications. Their focus on enhancing test accuracy and user experience is complemented by comprehensive after-sales support. Innovations in assay formats and sampling technologies further position QL Biotech as a leader in the diagnostic field, highlighting the manufacturer's dedication to advancing healthcare diagnostics.
- Healthcare Providers' Perspectives on Using Flu Antigen Tests
Interviews with healthcare providers reveal a strong preference for Flu Antigen Tests manufactured by QL Biotech due to their efficiency and reliability. The streamlined testing process and fast results allow clinicians to make informed decisions swiftly, a critical factor during peak influenza seasons. Providers also appreciate the manufacturer's attention to user-friendly design and detailed instructions, facilitating seamless integration into routine diagnostic workflows. These perspectives underscore the importance of manufacturer-provider collaboration in enhancing clinical outcomes.
- Impact of Rapid Flu Testing on Patient Management Strategies
Rapid Flu Antigen Tests, such as those by QL Biotech, significantly influence patient management strategies by enabling timely diagnosis and treatment initiation. By reducing diagnostic delays, these tests support healthcare providers in administering antiviral interventions within the optimal therapeutic window. Literature reviews corroborate the positive impact of rapid testing on patient outcomes, emphasizing reduced illness duration and transmission rates. The strategic incorporation of rapid tests into clinical protocols continues to shape contemporary healthcare practices.
- Navigating Test Sensitivity and Specificity: Insights for Providers
Understanding the nuances of test sensitivity and specificity is crucial for healthcare providers using Flu Antigen Tests. Despite advancements, rapid tests can sometimes yield false negatives, presenting challenges in clinical interpretation. Therefore, it is recommended to use these tests alongside clinical evaluations and, where necessary, confirmatory molecular assays. QL Biotech, as a manufacturer, provides comprehensive resources to aid providers in navigating these complexities, ensuring that diagnostic decisions are supported by robust data and clinical context.
- Manufacturers and the Future of Influenza Diagnostics
The future of influenza diagnostics is poised for transformation as manufacturers like QL Biotech innovate with more accurate and versatile testing platforms. The integration of digital technologies, such as AI-assisted result interpretation and telehealth compatibility, promises to enhance test usability and accessibility. As healthcare landscapes evolve, manufacturers are expected to play a pivotal role in driving the adoption of these cutting-edge solutions, aligning with global health goals of timely and precise disease detection.
- Understanding Regulatory Compliance in Diagnostic Manufacturing
Regulatory compliance remains a cornerstone for manufacturers like QL Biotech in the diagnostic space. Adherence to standards such as CE and ISO13485 is critical in ensuring test quality and safety, fostering trust with healthcare providers and end-users. Continuous collaboration with regulatory bodies also influences the development of new test parameters, optimizing diagnostic accuracy and reliability. By aligning product development with regulatory expectations, QL Biotech demonstrates a commitment to ethical and scientific excellence.
- Expanding Flu Antigen Testing Beyond Clinical Settings
The accessibility of Flu Antigen Tests extends beyond traditional clinics, with manufacturers now focusing on at-home testing solutions. QL Biotech is at the forefront of this shift, designing test kits that maintain high performance standards even in non-clinical environments. This expansion supports public health initiatives aimed at decentralized testing, increasing detection coverage and facilitating early intervention strategies. As manufacturers innovate towards greater accessibility, the potential to improve population-level health outcomes becomes more attainable.
- Collaborative Efforts in Diagnostic Test Innovation
Partnerships between manufacturers, researchers, and healthcare institutions drive significant advancements in diagnostic test innovation. QL Biotech collaborates with leading experts to refine test technologies and explore novel applications beyond traditional pathogen detection. These collaborative efforts often result in rapid prototyping and validation, ensuring that cutting-edge diagnostics are readily available to meet emerging health challenges. The synergy between multiple stakeholders accelerates the translation of research findings into practical healthcare solutions.
Image Description