Manufacturer HIV AG Rapid Test Device
Product Main Parameters
| Parameter | Specification |
|---|---|
| Sample Type | Whole Blood/Serum/Plasma |
| Detection Time | Less than 30 minutes |
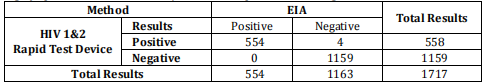
| Sensitivity | 99.9% |
| Specificity | 99.6% |
Common Product Specifications
| Component | Details |
|---|---|
| Test Devices | Included |
| Disposable Specimen Droppers | Included |
| Buffer | Included |
| Package Insert | Included |
Product Manufacturing Process
The manufacturing process of the HIV AG Rapid Test involves precision engineering to ensure accuracy and reliability. Following stringent quality control measures, the test devices are pre-coated with recombinant HIV antigens and the P24 antibody. The preparation of buffers and droppers is subjected to standardization to maintain consistency. The test strips are carefully assembled to facilitate accurate capillary action. Manufacturing practices are aligned with ISO standards and are routinely audited to meet compliance. This ensures that each batch of HIV AG Rapid Test devices produced by the manufacturer adheres to high standards of sensitivity and specificity, critical for effective HIV screening.
Product Application Scenarios
The HIV AG Rapid Test is invaluable in diverse applications such as clinics, hospitals, and mobile health units, especially in resource-constrained settings. Its rapid turnaround time allows for immediate decision-making in emergency care. It is particularly effective in outreach programs targeting high-risk populations, offering a reliable diagnostic tool without the need for extensive laboratory infrastructure. The test's ease of use empowers healthcare workers to conduct screenings widely, thus enhancing early detection and intervention efforts against HIV/AIDS. Such wide-ranging applicability highlights the test's role in driving public health outcomes.
Product After-sales Service
Our after-sales service includes comprehensive technical support and guidance for optimal test utilization. Customer service representatives are available 24/7 to address queries and provide solutions for any operational issues. The manufacturer offers a warranty and replacements for defective units, ensuring customer satisfaction.
Product Transportation
The products are packaged in tamper-proof boxes, ensuring they remain intact during transit. We partner with reliable logistics providers to offer timely delivery, with options for expedited shipping available. Strict temperature controls are maintained during transportation to preserve product integrity.
Product Advantages
- Fast and reliable results in under 30 minutes.
- High sensitivity and specificity for accurate detection.
- Usable in diverse settings without extensive training.
- Cost-effective and scalable for large populations.
Product FAQ
- What is the principle behind the HIV AG Rapid Test?
The HIV AG Rapid Test, manufactured with stringent quality controls, operates on lateral flow immunoassay technology. It detects HIV-1/2 antibodies and the p24 antigen in blood, serum, or plasma samples, providing rapid results. - How soon can the HIV AG Rapid Test detect HIV after exposure?
The test can identify the p24 antigen approximately 2-4 weeks post-exposure, significantly earlier than antibody-only tests, making it crucial for early diagnosis. - What is the shelf life of the HIV AG Rapid Test?
The test has a shelf life of up to 24 months, provided it is stored as per the manufacturer’s guidelines under optimal temperature conditions. - Can the test be used for self-testing at home?
While designed for rapid and accessible testing, it is recommended that the HIV AG Rapid Test be administered by healthcare professionals to ensure accurate interpretation. - Is additional equipment required for the HIV AG Rapid Test?
The manufacturer provides necessary components like test devices and droppers, but specimen collection containers, lancets, and timers need to be acquired separately. - How should a positive result be handled?
In the event of a positive result, it is crucial to follow up with confirmatory tests in a certified laboratory, as advised by healthcare professionals. - How should the HIV AG Rapid Test be stored?
To maintain efficacy, store the test kits at room temperature, away from direct sunlight, and within the temperature range provided by the manufacturer. - What makes the HIV AG Rapid Test a preferred choice?
Its rapid result delivery, combined with high accuracy, specificity, and versatility across various settings, makes it a preferred choice for early HIV detection. - Are there any known limitations of the test?
The HIV AG Rapid Test might yield false-positive results; hence, confirmatory testing is recommended. Also, early-stage infection can occasionally escape detection due to the ‘window period’. - What should be done if a test device appears damaged?
Discontinue use and contact customer services immediately for replacement as per the manufacturer's after-sales support policy.
Product Hot Topics
- How does the HIV AG Rapid Test contribute to public health?
By providing rapid and accurate results, the HIV AG Rapid Test empowers healthcare providers to initiate timely interventions, curbing the spread of HIV. Its accessibility promotes widespread testing, particularly in underserved regions, aiding in comprehensive HIV management and prevention strategies. - What are the implications of the HIV AG Rapid Test for rural healthcare?
In rural settings, where laboratory facilities are scarce, the HIV AG Rapid Test, manufactured for ease of use and portability, offers an indispensable solution. Its rapid deployment and minimal training requirements ensure that rural healthcare workers can effectively conduct large-scale screenings. - How is the HIV AG Rapid Test being integrated into national health programs?
Many national health programs adopt the HIV AG Rapid Test as part of their strategic testing initiatives. By aligning with healthcare goals, the manufacturer supports programs aiming to achieve global targets for HIV reduction, providing a robust tool for early detection and treatment pathway development. - What future enhancements are expected in HIV rapid testing?
Future advancements may involve integrating digital technology for result analysis and record-keeping. The manufacturer is exploring innovations to enhance sensitivity further and incorporate multi-disease testing capabilities in a single device, broaden its applicability, and improve healthcare outcomes. - How does the test promote awareness and early detection on a global scale?
Globally, awareness campaigns often leverage the rapid and reliable results of the HIV AG Rapid Test. The manufacturer collaborates with international health organizations to deploy these tests, emphasizing early detection as a pivotal strategy in combating the global HIV epidemic. - Why is the antigen detection in HIV AG Rapid Test crucial during early infection?
The inclusion of p24 antigen detection enables the identification of HIV sooner than antibody-only tests. This early detection is critical in initiating early treatment and reducing transmission risks, particularly during the acute phase of infection when viral loads are highest. - What role does the HIV AG Rapid Test play in community-based screenings?
Community screenings benefit from the rapid delivery and high accuracy of the HIV AG Rapid Test, facilitating immediate counseling and linkage to care. Its manufacturer emphasizes community engagement to improve testing uptake and ensure comprehensive coverage. - How does the manufacturer ensure the reliability of the HIV AG Rapid Test?
Strict quality control and adherence to international standards are at the core of the manufacturer’s production process. Continuous R&D efforts ensure that each batch meets high-performance metrics, offering healthcare professionals confidence in its reliability. - What economic benefits does the HIV AG Rapid Test offer to health systems?
Cost-effective and requiring minimal infrastructure, the HIV AG Rapid Test alleviates the financial burden on health systems. Its use as an initial screening tool streamlines the diagnostic process, reducing the reliance on expensive laboratory tests. The manufacturer’s focus on affordability without compromising quality supports sustainable healthcare delivery. - How does the test address testing disparities in low-resource settings?
In low-resource settings, the HIV AG Rapid Test’s straightforward administration and portability are crucial. The manufacturer partners with NGOs and health organizations to distribute test kits, ensuring equitable access and addressing testing disparities in these regions.
Image Description