Manufacturer of Malaria P. F. Test Cassette - QL Biotech
Product Main Parameters
| Parameter | Details |
|---|---|
| Detection Method | Immunochromatographic assay |
| Target Antigen | HRP2 |
| Sample Type | Whole Blood |
| Results Time | 15-20 minutes |
Common Product Specifications
| Specification | Details |
|---|---|
| Storage Temperature | 2-30°C |
| Kit Components | Test devices, droppers, buffer, package insert |
Product Manufacturing Process
Malaria P. F. Test Cassettes by manufacturers like QL Biotech undergo a stringent process of production. The immunochromatographic assay technology used requires precise assembly of components including conjugate pads with specific antibodies, nitrocellulose membranes, and control regions. Quality control at each stage ensures the sensitivity and specificity of the test, crucial for reliable results. Research highlights manufacturing excellence as a key factor in the effective deployment of these cassettes in resource-limited settings. This technology has revolutionized malaria diagnostics, offering a robust solution in the fight against the prevalent P. falciparum strain.
Product Application Scenarios
In endemic regions, the Malaria P. F. Test Cassette plays a pivotal role in immediate diagnosis of Plasmodium falciparum infections. Its application in remote clinics, field hospitals, and mobile units greatly enhances the ability of healthcare providers to identify and treat infected individuals swiftly. A study reflects its widespread acceptance due to ease of use and rapid results, crucial in high-risk communities. The practicality of these test cassettes makes them invaluable in proactive malaria management, supporting both healthcare professionals and at-risk populations in reducing transmission and mortality rates.
Product After-Sales Service
QL Biotech offers comprehensive after-sales support for their Malaria P. F. Test Cassette. This includes customer service to address inquiries, product training sessions, and technical support for troubleshooting. Replacement of defective products and guidance on proper test procedures ensure customer satisfaction and product efficacy.
Product Transportation
Transportation of the Malaria P. F. Test Cassette is managed under controlled conditions to preserve product integrity. Secure packaging protects from environmental factors, and distribution partners ensure timely delivery to both domestic and international customers, complying with stringent logistics standards.
Product Advantages
- Rapid and Accurate: Results within 15-20 minutes, ensuring timely diagnosis.
- Easy to Use: Requires minimal training, suitable for use in diverse settings.
- Portable: Compact design facilitates use in rural and mobile clinics.
Product FAQ
- Q1: How accurate is the Malaria P. F. Test Cassette?
As a manufacturer, QL Biotech ensures that the Malaria P. F. Test Cassette is rigorously tested for high accuracy, although factors like low parasite load can affect results.
- Q2: Can the test differentiate between Plasmodium species?
No, the Malaria P. F. Test Cassette specifically targets Plasmodium falciparum HRP2 antigen and does not detect other species.
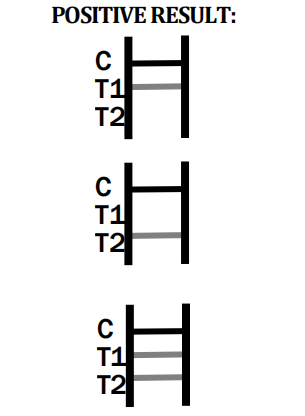
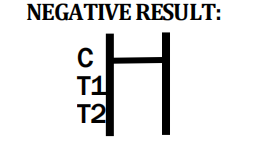
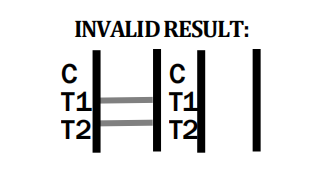
- Q3: What should I do if the control line does not appear?
In absence of the control line, the test is invalid. As a manufacturer, we advise retesting with a new cassette following package instructions.
- Q4: Is the test reusable?
Each Malaria P. F. Test Cassette is designed for single use to ensure accurate results and must be disposed of after testing.
- Q5: What is the shelf life of the test kits?
The manufacturer recommends use of the Malaria P. F. Test Cassette within the expiration date labeled on the packaging, under proper storage conditions.
- Q6: Can the test detect recent infections post-treatment?
Due to antigen persistence, results may remain positive post-treatment; further clinical assessment is advised for these cases.
- Q7: What volume of blood is required for testing?
The test requires approximately 50 μL of blood, ensuring adequate sample without excessive collection.
- Q8: How should the test kits be stored?
Store the Malaria P. F. Test Cassette between 2-30°C, away from direct sunlight and moisture, ensuring optimal storage conditions as advised by the manufacturer.
- Q9: Is additional equipment needed for the test?
No special equipment is required; the test includes all necessary components for sample collection and analysis.
- Q10: What is the ideal environment for performing the test?
Perform the test in a clean, dry location at room temperature to ensure the manufacturer's designated performance standards are met.
Product Hot Topics
Topic 1: The Role of RDTs in Malaria Endemic Regions The introduction of rapid diagnostic tests like the Malaria P. F. Test Cassette by leading manufacturers has significantly changed malaria management. The swift diagnostics supported by this tool have empowered health workers, decreased malpractice in treating fever-related illnesses, and ensured correct use of antimalarial drugs. As a result, healthcare systems witness improved resource allocation and patient outcomes, contributing to global commitments towards eradicating malaria.
Topic 2: Understanding the HRP2 Antigen and Its Importance The HRP2 antigen detection by the Malaria P. F. Test Cassette is a breakthrough in identifying P. falciparum infections. This antigen, produced by the parasite, serves as a distinct marker for diagnosis. However, genetic variations affecting HRP2 production present challenges that manufacturers are working to address through ongoing research, ensuring these tools continue to deliver optimal accuracy.
Topic 3: Combating Malaria with Innovative Diagnostic Tools In the battle against malaria, innovative diagnostic tools like the Malaria P. F. Test Cassette produced by manufacturers are indispensable. These tools speed up diagnosis and treatment, allowing health initiatives to focus on prevention strategies and reducing disease transmission. The widespread deployment of these tests underpins success stories in malaria control and highlights the need for sustained technology advancements.
Topic 4: Challenges in Malaria Diagnosis and How RDTs Assist Malaria diagnosis challenges are met with improved solutions such as the Malaria P. F. Test Cassette. While traditional methods require skilled personnel and equipment, the ease of use and quick results of RDTs enable broader diagnostic coverage. Health systems now reach communities with limited access, reflecting the manufacturer's commitment to addressing global health challenges through adaptive innovations.
Topic 5: The Economic Impact of Rapid Malaria Testing The economic benefits of rapid malaria testing with devices like the Malaria P. F. Test Cassette are significant. By reducing the time and cost of diagnosis, these tests allow for effective resource allocation in healthcare. Manufacturers contribute to economic growth in malaria-prone areas through improved workforce health and reduced disease burden, emphasizing the broader societal impact of enhanced diagnostic tools.
Topic 6: Future of Malaria Testing Technology The future of malaria testing is promising with continued advancements in rapid diagnostic tests like the Malaria P. F. Test Cassette. Manufacturers are researching ways to enhance sensitivity and specificity, adapting to genetic variations in parasite strains. As these technologies evolve, they promise more comprehensive malaria control solutions, supporting global eradication goals.
Topic 7: How Malaria P. F. Test Cassettes Support Health Workers Health workers in endemic areas rely heavily on tools like the Malaria P. F. Test Cassette to streamline diagnostic processes. Manufacturers equip these professionals with reliable tests, facilitating prompt decision-making and treatment, which are crucial in areas with high disease prevalence. This diagnostic support helps reduce malaria-related morbidity and mortality effectively.
Topic 8: Addressing False Positives in Malaria RDTs False-positive results in malaria RDTs, including those from the Malaria P. F. Test Cassette, pose challenges in clinical settings. Understanding antigen persistence and production variability allows manufacturers to enhance test designs, aiming to minimize inaccuracies. Continuous innovation and clinical trials are essential to refine diagnostic accuracy, ensuring better patient care and health outcomes.
Topic 9: Educational Initiatives on Using Malaria Test Kits Educational programs by manufacturers focus on training health workers in using the Malaria P. F. Test Cassette. These initiatives emphasize correct test procedures, interpreting results accurately, and avoiding common pitfalls. Comprehensive training not only maximizes test effectiveness but also reinforces confidence in the technology among healthcare providers.
Topic 10: Innovations in Malaria Test Kit Distribution Distribution strategies for the Malaria P. F. Test Cassette are being optimized by manufacturers to ensure widespread access. By leveraging local partnerships and advanced logistic frameworks, these test kits reach underserved areas promptly. Reinforced by innovative distribution methods, manufacturers enhance global efforts to expand diagnostic reach and improve malaria intervention success.
Image Description