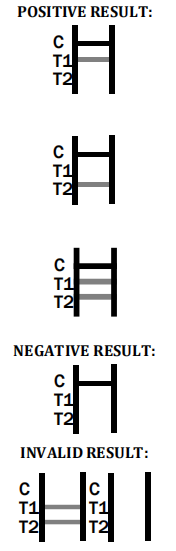Manufacturer Typhoid AG Rapid Test Device
Product Main Parameters
| Parameter | Details |
|---|---|
| Type | Rapid Test Device |
| Specimen | Feces |
| Reading Time | 10 minutes |
| Storage | 2‐30°C |
Common Product Specifications
| Specification | Description |
|---|---|
| Package | 20 Tests |
| Components | Test devices, droppers, buffer, package insert |
Product Manufacturing Process
Referencing authoritative papers, the manufacturing of the Typhoid AG test involves a precise immunoassay assembly, ensuring high specificity and sensitivity. The process includes the conjugation of antibodies to colloid gold, followed by meticulous assembly on nitrocellulose membranes. Quality control processes are integral to maintaining assay performance. Research emphasizes continuous refinement for enhanced detection capabilities.
Product Application Scenarios
According to authoritative studies, the Typhoid AG test is applicable in various scenarios, particularly in low-resource settings. It facilitates point-of-care testing, enabling rapid diagnosis crucial for early intervention. The simplicity of the test allows its use by non-specialist personnel, enhancing its utility in public health initiatives targeting typhoid fever endemic regions.
Product After-sales Service
Our manufacturer ensures comprehensive after-sales support including troubleshooting assistance, result verification services, and customer training for optimal product usage.
Product Transportation
Products are shipped under controlled conditions, maintaining storage temperature to ensure integrity upon delivery. Logistics support further assures timely distribution.
Product Advantages
- Rapid results within 10 minutes
- Easy to use for non-specialists
- Applicable in diverse settings
- Reliable detection of S. typhoid and S. Para typhi
Product FAQ
- What is the Typhoid AG test? The Typhoid AG test is a rapid immunoassay for identifying S. typhoid and S. Para typhi antigens in feces, providing quick results to aid in diagnosis.
- Who is the manufacturer? The test is manufactured by Zhejiang QL Biotech Co., Ltd, specialists in diagnostic reagents with over 20 years of experience.
- How accurate is the test? The manufacturer has optimized the test to offer high sensitivity and specificity, though confirmatory testing may be recommended in certain cases.
- What are the storage conditions? The test should be stored at temperatures between 2‐30°C to maintain its efficacy until the expiration date.
- Can the test be used in field conditions? Yes, the test's rapid results and minimal equipment requirements make it suitable for field use, particularly in resource-limited settings.
- Is training required to use the test? The test is designed to be user-friendly, with clear instructions provided, minimizing the need for extensive training.
- What are the components provided? Each kit includes test devices, droppers, buffer solution, and a detailed package insert.
- How should specimens be collected? Specimens are collected using the provided applicator stick from multiple sites in the feces to ensure accurate representation.
- What is the role of the control band? The control band ensures the validity of the test. A visible control band confirms a proper procedure was followed.
- Are there any limitations to the test? As with any diagnostic test, there may be limitations such as potential false positives or negatives, highlighting the importance of comprehensive diagnostic strategies.
Product Hot Topics
- Is rapid testing the future of diagnostics? Rapid testing like the Typhoid AG is revolutionizing diagnostics by enabling prompt and accessible healthcare solutions, especially in remote areas, enhancing disease management and control.
- How does Typhoid AG testing impact public health? By providing quick and reliable results, the manufacturer of Typhoid AG tests significantly contribute to public health efforts in controlling and managing typhoid fever outbreaks.
- What innovations are manufacturers introducing in Typhoid AG testing? Manufacturers are continuously refining test sensitivity and specificity, incorporating advanced materials and processes to ensure reliable diagnostics even in challenging settings.
- How does Typhoid AG testing compare to traditional methods? Compared to traditional methods like blood culture, Typhoid AG offers speed and simplicity, making it a valuable tool for immediate clinical decisions, especially where resources are limited.
- What are the challenges faced by Typhoid AG test manufacturers? Manufacturers face challenges such as ensuring batch consistency, minimizing cross-reactivity, and adapting to evolving strains, necessitating ongoing research and quality control.
- How do manufacturers address test reliability? Manufacturers implement rigorous quality control measures and validation processes to ensure each Typhoid AG test meets stringent reliability standards before reaching the market.
- What role does Typhoid AG testing play in endemic regions? In endemic regions, the manufacturer’s Typhoid AG tests are crucial for early detection, helping to curb the spread of the disease through timely and targeted interventions.
- How accessible are Typhoid AG tests in rural areas? Designed for minimal infrastructure use, these tests provide essential diagnostic capabilities in rural areas, assisting healthcare providers with immediate patient management.
- Are there plans for future enhancements in Typhoid AG tests? Manufacturers are exploring improvements through technological advancements and feedback from healthcare professionals, aiming to enhance user experience and diagnostic accuracy.
- Can Typhoid AG tests be integrated into broader healthcare systems? Integration into healthcare systems allows for comprehensive disease monitoring and management, leveraging quick diagnostics to improve patient outcomes and reduce healthcare burdens.
Image Description