New Coronavirus Quick Check Factory: SARS-COV-2/Flu AB
Product Main Parameters
| Brand | QL |
|---|---|
| Certificate | CE |
| Specimen | Nasopharyngeal swabs/Nasal swab |
| Pack | 20T |
| Reading Time | 10 minutes |
| Contents | Cassette, Buffer, Package insert |
| Storage | 2-30℃ |
| Shelf Life | 2 years |
Product Manufacturing Process
As highlighted in authoritative studies, the manufacturing process of rapid diagnostic tests involves several critical stages: antigen selection and cloning, antibody production, and conjugation. The antigens are carefully selected to represent the virus accurately, ensuring specificity. Antibodies are produced via hybridoma technology, creating monoclonal antibodies that are then conjugated to visual markers for the test strip. These components are assembled in a controlled environment to ensure quality and reliability. The production process is designed to maximize the sensitivity and specificity of the test, crucial for accurate detection of the SARS-CoV-2 and Influenza viruses. Through continuous research and improvements, our factory ensures that each test produced adheres to the highest standards, contributing significantly to global testing capabilities.
Product Application Scenarios
According to academic research, rapid tests play a crucial role in various application scenarios. In healthcare settings, they are used to quickly identify infections in symptomatic individuals, helping to manage patient flow and resources effectively. In schools and workplaces, they offer a means to monitor and control outbreaks, ensuring a safer environment. Public health officials use rapid tests for mass screening, particularly in high-risk communities, to contain potential outbreaks. The ability to provide results in minutes makes these tests invaluable in emergency response situations, significantly reducing the time to implement appropriate interventions. By utilizing our New Coronavirus Quick Check, users in diverse scenarios can make informed decisions promptly, enhancing public health outcomes.
Product After-Sales Service
Our factory offers comprehensive after-sales support, ensuring customers achieve optimal performance from the New Coronavirus Quick Check. This includes access to a dedicated support team available to resolve technical issues or answer queries. We also provide online resources, such as troubleshooting guides and user manuals, to aid in the correct use of our products. Additionally, we offer replacement services for defective items, ensuring customer satisfaction and trust in our brand.
Product Transportation
Transportation of the New Coronavirus Quick Check is conducted under stringent conditions to maintain product integrity. Our packages are designed to withstand varied climatic conditions and are labeled with clear handling instructions. We collaborate with trusted logistics partners to ensure timely delivery across regions, with tracking services available for real-time updates. Our logistics strategy prioritizes both efficiency and safety, ensuring that each batch arrives in optimal condition.
Product Advantages
Our New Coronavirus Quick Check factory delivers several advantages:
- Rapid Results: Obtain results in just 10 minutes.
- High Accessibility: Suitable for use in various settings, including clinics and at-home testing.
- Cost-Effective: More affordable than traditional lab-based tests.
Product FAQ
- What is the turnaround time for results?
Our New Coronavirus Quick Check offers results in just 10 minutes, making it one of the fastest diagnostic tools available in the market. This speed is particularly beneficial for urgent testing needs, such as in healthcare facilities or during outbreak investigations. The quick turnaround allows for immediate decision-making, helping to control the spread of infections effectively.
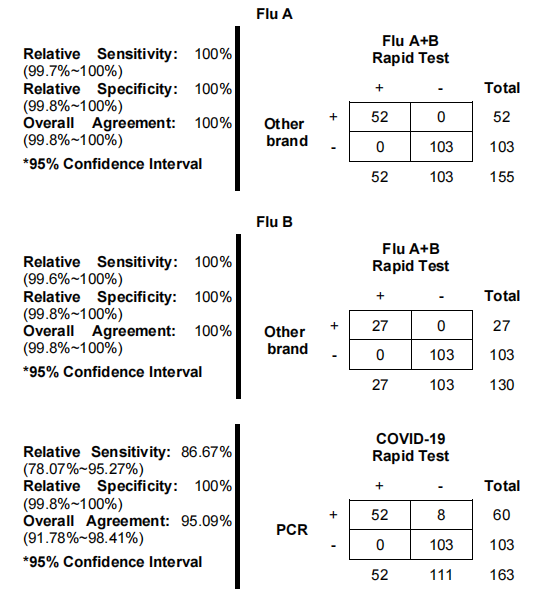
- How accurate is the test?
The test is designed to meet high standards of accuracy, incorporating advanced antigens and monoclonal antibodies for reliable detection. While no test can guarantee 100% accuracy, our factory's rigorous quality control processes ensure that each kit provides dependable results, making it a trusted tool for rapid diagnosis of SARS-CoV-2 and Influenza AB.
- Can the test be used at home?
Yes, the New Coronavirus Quick Check is designed for easy use in both professional and home settings, with clear instructions provided. However, it is important to follow the instructions carefully to ensure accurate results. For those using it at home, consulting with a healthcare professional for interpretation of results is recommended.
- What specimens are required for testing?
Our test requires nasopharyngeal or nasal swabs for sample collection. These types of specimens are known for their reliability in detecting respiratory pathogens such as SARS-CoV-2 and Influenza viruses. Proper collection and handling of samples are essential to ensure the accuracy of the test results.
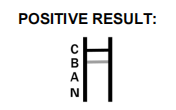
- What should I do if I get a positive result?
If you receive a positive result, it is important to contact your healthcare provider immediately for further evaluation and to discuss the next steps, including potential isolation to prevent virus transmission. Confirmatory testing and medical advice should be sought to ensure appropriate management of your health condition.
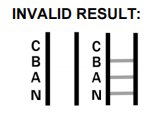
- What should I do if my test is invalid?
An invalid result may occur if the control band does not appear. In such cases, it is essential to repeat the test with a new kit following the instructions carefully. If the issue persists, please discontinue use and contact our support team for assistance.
- How should the test be stored?
The New Coronavirus Quick Check should be stored between 2-30℃, away from direct sunlight and moisture, to maintain its effectiveness. Proper storage is key to ensuring the test's performance and longevity over its two-year shelf life.
- Is the test CE certified?
Yes, our test is CE certified, indicating compliance with European standards for health, safety, and environmental protection. This certification assures users of the test's quality and efficacy, providing confidence in its results.
- Can the test detect new virus variants?
The New Coronavirus Quick Check is designed to detect primary antigens shared by known variants. However, continuous development and updates are necessary to adapt to new strains. Our factory is committed to monitoring emerging variants and enhancing the test's capabilities accordingly.
- How is customer support handled?
Our factory provides a dedicated customer support service to address any queries or issues related to the New Coronavirus Quick Check. Customers can reach out through multiple channels, including phone, email, and online chat, ensuring efficient resolution of concerns.
Product Hot Topics
- Discussion on Rapid Test Accuracy
As the need for quick diagnostic tools grows, debates around rapid test accuracy intensify. Our New Coronavirus Quick Check factory is committed to delivering high-accuracy tests, but understanding the balance between speed and sensitivity is crucial for users. While rapid tests offer fast results, users should be aware of the possibility of false positives or negatives and consult healthcare providers for comprehensive evaluations. Discussions on this topic emphasize the importance of context in test usage and the role of confirmatory testing in public health strategies. Keeping abreast of advancements and updates in rapid diagnostic technologies can enhance user perception and application of these critical tools.
- The Role of Rapid Tests in Pandemic Response
Rapid tests, such as those produced by our New Coronavirus Quick Check factory, have become indispensable in the global pandemic response. They provide essential data on virus prevalence and transmission, enabling efficient public health interventions. These tests facilitate large-scale screening in various environments, from healthcare settings to community gatherings, helping to manage outbreaks and inform policy decisions. As new infectious threats emerge, rapid tests will continue to be pivotal in early detection and containment efforts, underscoring their importance in modern epidemiological practices. Engaging in discussions about this role can spread awareness and appreciation for rapid testing's impact on global health.
Image Description