New Coronavirus Quick Check - QL Biotech's Combo Rapid Test Device
PRODUCT SPECIFICATION
|
Brand |
QL |
Certificate |
CE |
|
Specimen |
Nasopharyngeal swabs/ Nasal swab |
Pack |
20T |
|
Reading Time |
10 minutes |
Contents |
Cassette , Buffer ,Package insert |
|
Storage |
2-30℃ |
Shelf Life |
2 years |
INTERPRETATION OF RESULTS
Same test procedure As SARS-COV-2 Antigen Rapid Test Device (Swab), The result Should be read at 10 minutes, Do not interpret the result after 20 minutes.
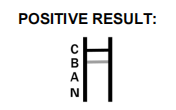
Possible Interpretation of Test Results:Flu B Positive:* A colored band appears in the control band region (C) and another colored band appears in the B region.

Flu A Positive:* A colored band appears in the control band region (C) and anothere colored band appears in the A region.

Flu A+B Positive:* A colored band appears in the control band region (C) and two other colored bands appear in the A region and B regions, respectively.
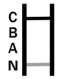
COVID-19 Positive:* A colored band appears in the control band region (C) and another colored band appears in the N region.

NEGATIVE RESULT:Only one colored band appears, in the control band region (C). No band appears in either test band region (A/B/N).
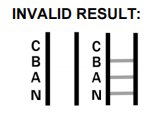
INVALID RESULT: Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor.
PERFORMANCE CHARACTERISTICS
Table: Flu A+B Rapid Test vs. other commercial brand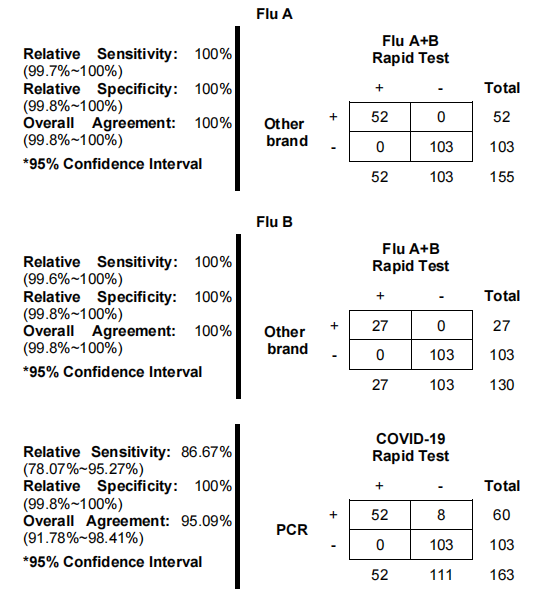
The accuracy of the New Coronavirus Quick Check's results is evident through its interpretation guidelines. Much like the SARS-COV-2 Antigen Rapid Test Device, the results should be read at 10 minutes. This ensures accuracy and dependability of the results. However, it's important not to interpret the results after 20 minutes as this could affect their reliability. In conclusion, QL Biotech's New Coronavirus Quick Check offers a quick, reliable, and easy-to-use solution for detecting SARS-COV-2/Influenza A+B. Trust this CE certified product for your testing needs and stay one step ahead in virus detection and control.


