Can I do a dengue test at home?
Introduction to Dengue Rapid Test Devices
● Overview of Dengue Virus and Its Impact
Dengue fever, a mosquito-borne viral disease, poses significant global health challenges, especially in tropical and subtropical regions. With millions of cases reported annually, early diagnosis and prompt treatment are crucial in controlling outbreaks and reducing mortality rates. Rapid diagnostic tools, such as the Dengue Test Kit, play an indispensable role in the timely detection and management of dengue infections.
● The Importance of Rapid Testing in Dengue Diagnosis
Rapid testing for dengue is pivotal due to the disease's rapid progression and the potential for severe complications, such as dengue hemorrhagic fever. Utilizing a Dengue Test Kit allows healthcare professionals to quickly diagnose and differentiate between dengue types, ensuring timely patient management and reducing the burden on healthcare systems.
Understanding the Dengue IgG/IgM Rapid Test
● What is the IgG/IgM Rapid Test?
The IgG/IgM rapid test is a lateral flow chromatographic immunoassay designed to simultaneously detect and differentiate IgG and IgM antibodies to the dengue virus. This diagnostic tool helps identify both current and past infections, providing a comprehensive overview of a patient’s serological status.
● Key Features and Benefits of the IgG/IgM Test
The IgG/IgM test offers several advantages, including speed, simplicity, and accuracy. It requires minimal technical expertise, making it accessible in low-resource settings. Additionally, the test yields results within 15-20 minutes, enabling swift clinical decision-making and appropriate intervention strategies. Wholesale Dengue Test Kit manufacturers provide affordable solutions, making these tests widely available to healthcare providers.
How the Dengue IgG/IgM Test Works
● The Principle Behind the IgG/IgM Rapid Test
The IgG/IgM rapid test operates on a simple principle: the antigen-antibody reaction. A sample of the patient's blood, serum, or plasma is placed on the test device. As the sample migrates along the strip, it encounters dengue antigens conjugated with colloidal gold particles. If antibodies are present, they bind to these antigens, forming visible colored lines, which indicate a positive result.
● Explanation of Test Components and Process
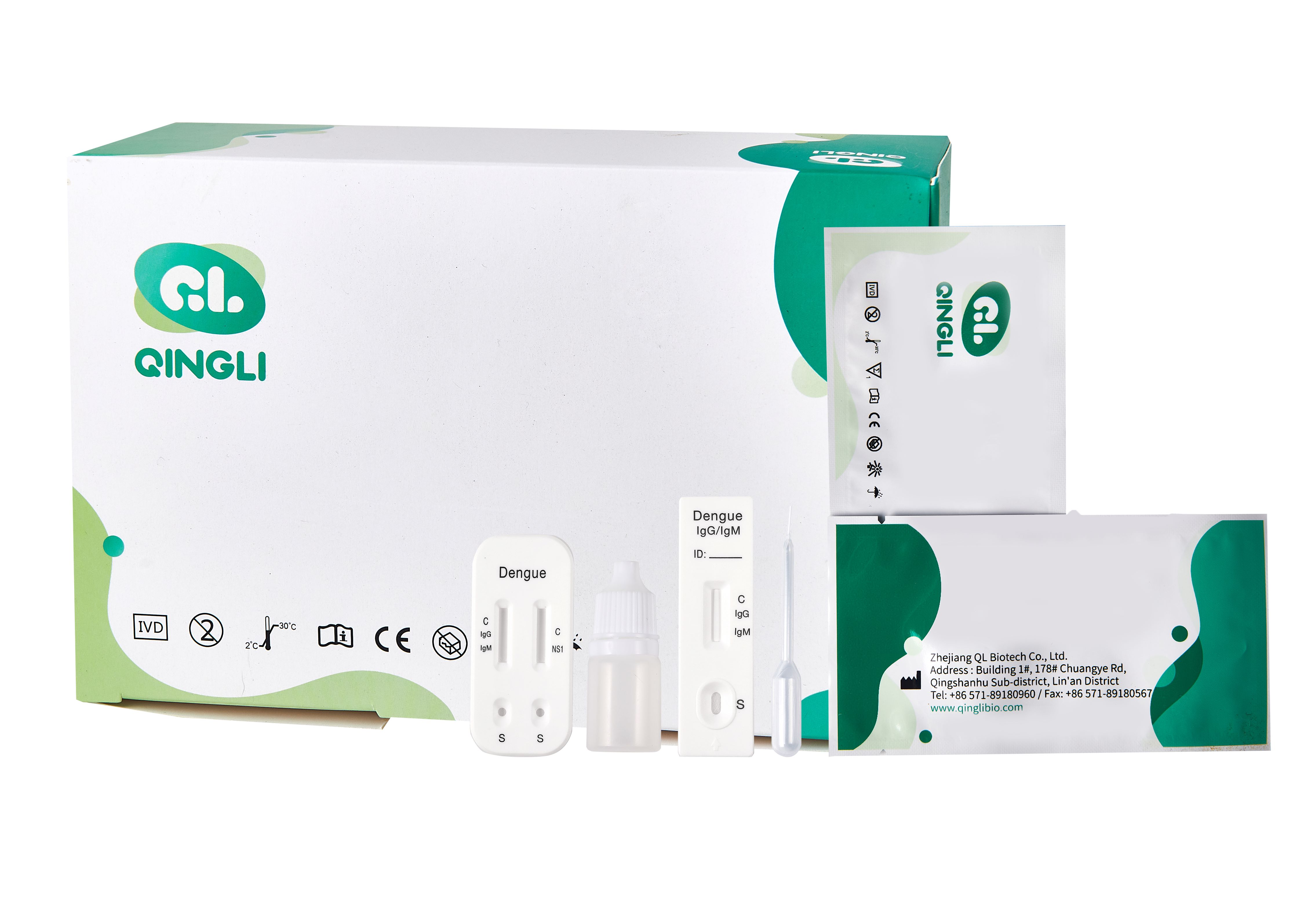
A standard Dengue IgG/IgM Test Kit contains a test cassette, a dropper, and a buffer solution. The test cassette has a sample well for applying the specimen and buffer, and a result window where results are displayed. Correct use and interpretation are crucial for reliable results, as outlined by Dengue Test Kit suppliers and manufacturers.
Conducting the IgG/IgM Test: Step-by-step Guide
● Preparing the Specimen and Test Components
Before commencing the test, it is essential to prepare all components. Ensure the specimen is appropriately collected and handled, and all test materials are at room temperature. Dengue Test Kit factories emphasize adherence to these guidelines to ensure accuracy.
● Detailed Assay Procedure for Whole Blood, Serum, and Plasma
For whole blood samples, use the provided dropper to transfer the sample into the test cassette's well, followed by buffer addition. For serum or plasma, the steps are similar but require specific volumes as per the manufacturer’s instructions. Results should be read within the designated time frame to ensure validity.
Introducing the Dengue NS1 Rapid Test Device
● Function of the NS1 Rapid Test in Diagnosis
The NS1 antigen test is a crucial diagnostic tool for early dengue detection, typically effective from the first day of fever onset. It identifies the presence of the non-structural protein 1 (NS1) antigen, a marker of active dengue infection, thus assisting in early diagnosis and timely intervention.
● Situations Where NS1 Testing is Recommended
NS1 testing is particularly useful during the acute phase of infection, within the first five days. It provides a reliable diagnostic option when antibody detection may be insufficient. Dengue Test Kit manufacturers stress the importance of early testing to enhance patient outcomes and outbreak management.
Step-by-step NS1 Test Procedure
● Assay Procedure for Whole Blood and Other Samples
The NS1 antigen test follows a similar procedure to the IgG/IgM test. Apply the patient’s sample to the test cassette, add the buffer, and wait for the results to appear in the designated window. Accurate adherence to instructions from wholesale Dengue Test Kit suppliers ensures the reliability of the test.
● Important Considerations for Accurate Test Results
The accuracy of the NS1 test depends on proper specimen handling, correct interpretation of results, and adherence to storage and procedural guidelines. Manufacturers recommend regular training for healthcare providers to maintain proficiency.
Combining IgG/IgM and NS1: The Combo Test
● Advantages of the IgG/IgM/NS1 Combo Test Device
The combination of IgG/IgM and NS1 tests into a single device enhances diagnostic efficiency, offering a comprehensive assessment of both acute and past dengue infections. This dual approach ensures rapid and accurate diagnosis, aiding in effective patient management.
● When to Use the Combo Test for Improved Diagnosis
Utilizing the combo test is beneficial throughout the dengue season and in clinical settings with high dengue prevalence. It provides a holistic diagnostic overview, enabling clinicians to tailor treatment strategies effectively.
Interpreting Results: Decoding the Assay
● Explanation of Possible Outcomes for IgG, IgM, and NS1 Tests
Each test component in the Dengue Test Kit yields distinct results. A positive IgM indicates a recent infection, while a positive IgG suggests past exposure or late-stage current infection. Positive NS1 results confirm an active infection. Recognizing these outcomes is crucial for diagnosis and treatment planning.
● Understanding Positive, Negative, and Invalid Results
Valid test results must display a control line, ensuring the test's integrity. The absence of this line indicates an invalid test, necessitating retesting. Positive and negative results guide clinical decisions, so proper interpretation is vital.
Limitations and Confirmatory Testing
● Recognizing the Limitations of Rapid Tests
While rapid tests are invaluable, they have limitations, such as potential cross-reactivity and reduced sensitivity in later infection stages. Understanding these limitations is essential for accurate diagnosis.
● Importance of Confirmatory Testing and Clinical Correlation
Confirmatory testing, such as PCR or ELISA, is recommended to validate rapid test results. Clinical correlation with patient history and symptomatology ensures comprehensive diagnosis and optimal patient care.
Storage, Precautions, and Best Practices
● Proper Storage Conditions for Test Kits
Dengue Test Kit factories emphasize the importance of storing kits within recommended temperature ranges to preserve efficacy. Improper storage can compromise test performance and accuracy.
● Tips for Ensuring Accuracy and Preventing Errors in Testing
Adhering to manufacturer protocols, regular training, and meticulous attention to detail in test execution help mitigate errors. Dengue Test Kit suppliers advocate for these best practices to maintain high diagnostic standards.
Enterprise Introduction
Zhejiang QL biotech Co., Ltd is a company that focuses on producing and researching diagnostic reagents. Their management and R&D team have over 20 years of experience in product development and production within the IVD industry. The company's primary products include infectious disease products, cardiac marker detection products, DOA products, and tumor marker products. At QL biotech, core values such as integrity, commitment, respect, openness to change, and passion guide their mission to make everyday life easier for their customers and partners.