OEM Fecal H. Pylori AG Test Device - Rapid Detection Kit
PRINCIPLE
The HBsAg Rapid Test Device (Serum/Plasma) has been designed to detect the HBsAg through visual interpretation of color development in the strip. The membrane was immobilized with anti‐ HBsAg antibodies on the test region. During the test, the specimen is allowed to react with colored anti‐HBsAg antibodies colloidal gold conjugates, which were precoated on the sample pad of the test. The mixture then moves on the membrane by a capillary action, and interact with reagents on the membrane. If there were enough HBsAg in specimens, a colored band will from at the test region of the membrane. Presence of this colored band indicates a positive result, while its
absence indicates a negative result. Appearance of a colored band at the control region serves as a procedural control. This indicates that proper volume of specimen has been added and membrane wicking has occurred.
Product Detail
Brand: QL
Specimens: : Serum/Plasma
Reading time:Device:15 minutes./ Strip 10 minutes.
Pack:25 T
STORAGE: 2‐30°C
KIT COMPONENTS
1
Individually packed test devices
Disposable pipettes
Buffer
Package insert
2
Individually packed test strips
Disposable pipettes
Package insert
PROCEDURE
- HBsAg Rapid Test Strip
Bring tests, specimens and/or controls to room temperature (15‐30°C) before use.
1. Transfer 3 drop of specimen to a specimen reaction tube with a disposable pipette provided in the kit.
2. Remove the test from its sealed pouch and use it as soon as possible. To obtain a best result, the assay should be performed within one hour.
3. Hold the strip at the handle with the product name imprints. Do not touch the membrane part of the strip to avoid contamination.
4. Dip the test strip vertically in the specimen for at least 10‐15 seconds. Do not pass the maximum line (MAX) on the test strip when immersing the strip.
As the test begins to work, you will see color move across the membrane.
5. Take the strip out of the specimen afterwards and place it on a non‐absorbents flat surface.
Start the timer and wait for the colored line(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 20 minutes.
HBsAg Rapid Test Device
Bring tests, specimens and/or controls to room temperature (15‐30°C) before use.
1. Remove the test from its sealed pouch, and place it on a clean, level surface. Label the test with patient or control identification. To obtain a best result, the assay should be performed
within one hour
Transfer 2 drops of serum/plasma to the specimen well of the device with a disposable pipette provided in the kit, then add 1 drop of buffer and start the timer.
Avoid trapping air bubbles in the specimen well (S), and do not drop any solution in observation window.
As the test begins to work, you will see color move across the membrane.
2. Wait for the colored band(s) to appear. The result should be read at 15 minutes. Do not interpret the result after 20 minutes.
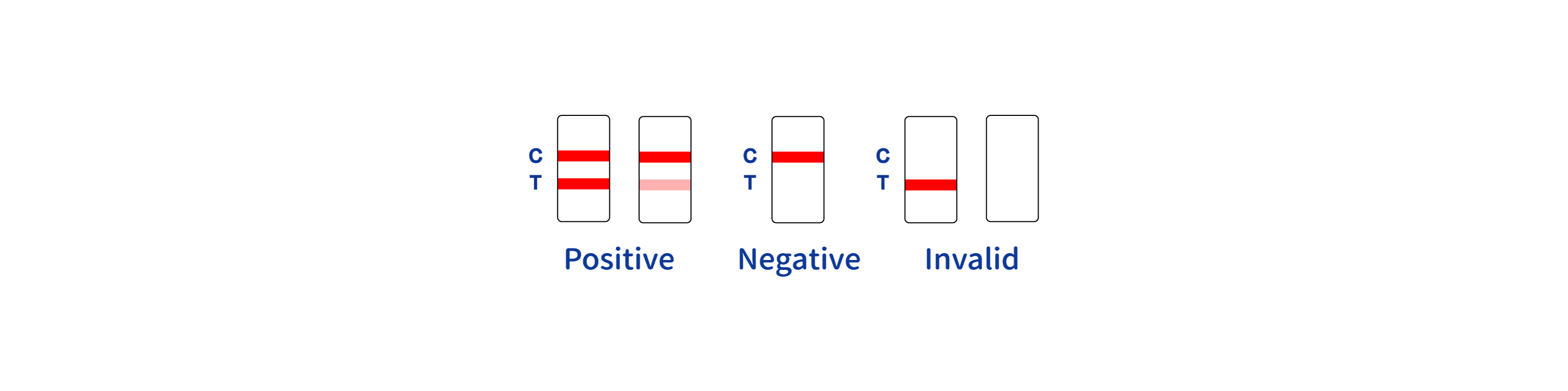
INTERPRETATION OF RESULTS
The OEM Fecal H. Pylori AG Test Device leverages advanced immunochromatographic techniques to provide healthcare professionals with swift, precise results. Unlike traditional methods that require extensive lab work and prolonged waiting periods, our test delivers outcomes within minutes. This rapid turnaround is crucial in clinical settings where time is of the essence, enabling prompt diagnosis and the initiation of appropriate treatment without delay. Our device’s user-friendly design ensures a straightforward testing process, requiring minimal training for operation. It is specifically formulated to detect H. pylori antigens with high sensitivity and specificity, reducing the risk of false positives or negatives that could impact patient care. As a leading provider of OEM diagnostic solutions, QL Biotech is dedicated to delivering products that adhere to the highest standards of quality and reliability. The OEM Fecal H. Pylori AG Test Device represents our commitment to these values, offering a cost-effective, efficient solution for healthcare providers worldwide. With our device, practitioners can enhance their diagnostic capabilities, ensuring better patient outcomes through the timely detection of H. pylori infections.