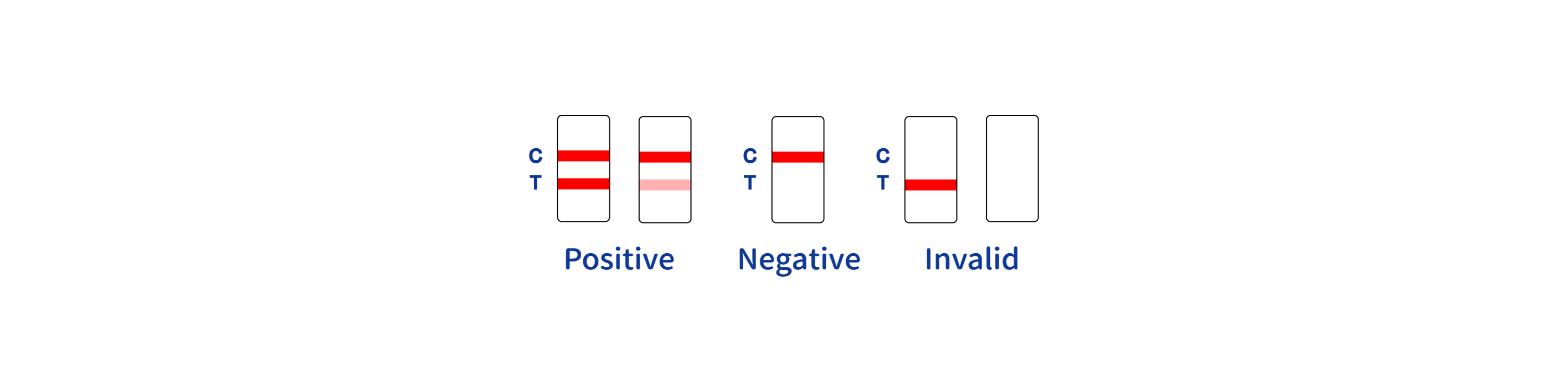
QL Biotech HIV P24 Rapid Test Manufacturer
Product Main Parameters
| Parameter | Details |
|---|---|
| Brand | QL Biotech |
| Specimen Type | Whole Blood/Serum/Plasma |
| Reading Time | Device: 10 minutes, Strip: 15 minutes |
| Pack Size | 25 Tests |
| Storage | 2-30°C |
Common Product Specifications
| Component | Details |
|---|---|
| Test Devices | Included |
| Disposable Droppers | Included |
| Buffer | Included |
| Package Insert | Included |
Product Manufacturing Process
The HIV P24 Rapid Test is produced adhering to strict quality control standards to ensure high reliability and accuracy. According to research, chromatographic immunoassays are enhanced by precise coating processes, minimizing false negatives and ensuring that the p24 antigen is effectively detected. Studies emphasize the importance of maintaining stringent environmental controls throughout production to preserve antigen and antibody stability. Furthermore, the incorporation of robust procedural controls like colored lines assists in validating test efficacy, as supported by authoritative sources in immunodiagnostics.
Product Application Scenarios
The HIV P24 Rapid Test is invaluable in clinical settings where rapid diagnosis is crucial for initiating early treatment protocols. As noted in public health studies, this test is highly effective in areas with limited access to advanced laboratory facilities, offering prompt and reliable results essential for targeted intervention. Deploying the test in high-prevalence regions supports large-scale screening efforts, aiding in the reduction of community transmission and improving patient outcomes, a strategy well-documented in healthcare management literature.
Product After-Sales Service
QL Biotech offers comprehensive after-sales support, including technical assistance, troubleshooting, and product replacement for defective units. Our dedicated customer service team is available to ensure optimal product performance and user satisfaction.
Product Transportation
All HIV P24 Rapid Test kits are shipped under controlled conditions to maintain their integrity. Adhering to international shipping standards, our logistics team ensures timely delivery, with all necessary precautions taken to protect the product during transit.
Product Advantages
- Early Detection: Enables identification of HIV infection weeks before antibody tests.
- Rapid Results: Provides results within 20 to 30 minutes.
- Ease of Use: Simple procedure suitable for diverse settings.
- Cost-Effective: Affordable for use in extensive screening campaigns.
Product FAQ
- What is the principle behind the HIV P24 Rapid Test?
The test uses a membrane-based immunoassay to detect HIV p24 antigens. It involves antibodies that bind specifically to the p24 protein if present, with a visual indicator confirming the presence of the antigen. - How quickly can results be obtained?
The HIV P24 Rapid Test typically delivers results within 20 to 30 minutes, allowing for timely clinical decision-making. - Is the test suitable for all populations?
Yes, it is designed for broad application, including in resource-limited settings, due to its ease of use and minimal requirements for specialized equipment. - What is the storage condition for the test kit?
The test should be stored at temperatures between 2 and 30°C to preserve its efficacy. - Does the test require confirmation?
Yes, positive results should be confirmed with additional tests such as nucleic acid tests (NAT) for accurate diagnosis. - Can the test be used for chronic infections?
The P24 antigen is most detectable in the early stages of infection; for chronic infections, supplementary tests are recommended. - Is the HIV P24 Rapid Test cost-effective?
Yes, it is a cost-effective option, ideal for widespread screening programs, particularly in high-prevalence areas. - What specimen types are required for the test?
The test can be conducted using whole blood, serum, or plasma samples. - Does the test require special training?
No, the test is user-friendly and can be performed by personnel without extensive laboratory training. - Is there an impact on public health?
Yes, early detection helps in initiating prompt treatment, reducing transmission rates, and improving patient prognosis.
Product Hot Topics
- Impact of Early HIV Detection on Patient Outcomes
The ability of the HIV P24 Rapid Test to detect HIV infection early plays a crucial role in patient outcomes. Early detection allows for the initiation of antiretroviral therapy, which significantly reduces viral load and improves immune function, leading to better long-term health. Studies have shown that patients diagnosed early can achieve near-normal life expectancy and reduced risk of transmitting the virus to others. The role of early testing in controlling the spread of HIV, particularly in high-risk populations, cannot be overstated. - Cost-Effectiveness of Rapid HIV Testing
The HIV P24 Rapid Test offers a cost-effective solution for large-scale HIV screening programs. Compared to laboratory-based tests, the rapid test is more affordable and provides immediate results, which is crucial in resource-limited settings. By facilitating early intervention, these tests can help reduce the healthcare burden associated with advanced HIV-related diseases. Additionally, the simplicity and low cost make it accessible to regions with limited healthcare infrastructure, highlighting its importance in global health initiatives. - The Role of HIV P24 Rapid Test in Resource-Limited Settings
The HIV P24 Rapid Test is particularly valuable in resource-limited settings where access to comprehensive laboratory facilities is constrained. Its ease of use, requiring minimal equipment and training, makes it ideal for field use, mobile clinics, and remote areas. By providing quick and accurate results, the test enables healthcare workers to make immediate clinical decisions, improving patient management and contributing to better public health outcomes. - Comparison of HIV P24 Rapid Test with Traditional Antibody Tests
The HIV P24 Rapid Test is advantageous over traditional antibody tests due to its ability to detect HIV infection earlier. While traditional tests require a longer window period for antibody development, the P24 rapid test identifies the p24 antigen, which appears sooner after infection. This early detection capability is critical for initiating timely treatment and reducing transmission. However, it is essential to confirm rapid test results with additional diagnostic tests for comprehensive patient management. - Understanding the Window Period in HIV Testing
The window period is a key consideration in HIV testing. The HIV P24 Rapid Test shortens this period by detecting the p24 antigen before antibodies are present. Typically, the antigen is detectable within 1 to 3 weeks post-infection, whereas antibody tests may take several weeks. This early detection is pivotal in managing HIV, as it allows for quicker treatment initiation, thus improving prognosis and reducing infection spread. - Optimizing HIV Screening with Rapid Testing
Incorporating the HIV P24 Rapid Test into screening strategies enhances early identification of HIV, crucial for controlling the epidemic. When combined with confirmatory tests, rapid testing offers a reliable method for screening high-risk populations, ensuring those infected receive prompt care. This approach is particularly effective in settings with high HIV prevalence, enabling healthcare systems to address the infection proactively and efficiently. - The Science Behind HIV P24 Antigen Detection
The HIV P24 Rapid Test operates by identifying the p24 antigen, a protein integral to the viral core of HIV. This antigen appears early in the infection process, making it an ideal target for early diagnosis. The test's immunoassay mechanism utilizes antibodies that selectively bind to the p24 antigen, with a visible color change indicating a positive result. This detection method is supported by extensive research, validating its accuracy and reliability in early HIV diagnosis. - Public Health Implications of Rapid HIV Testing
The widespread use of the HIV P24 Rapid Test has significant public health implications. By facilitating early detection, the test enables health authorities to implement timely interventions, reducing the spread of HIV. Early diagnosis allows for better resource allocation, targeted prevention programs, and improved patient outcomes, aligning with global health goals to curb the HIV epidemic. The test's role in early detection underscores its importance as a public health tool. - Challenges and Limitations of HIV P24 Rapid Testing
While the HIV P24 Rapid Test offers numerous advantages, it also has limitations such as a narrow detection window and possible false negatives in later stages of infection. It is most effective when used in conjunction with other diagnostic methods. Understanding these limitations is crucial for healthcare providers to make informed decisions and provide comprehensive care. Continuous advancements in testing technology aim to address these challenges, improving diagnostic accuracy and patient outcomes. - The Future of HIV Diagnostics and the Role of Rapid Testing
The evolving landscape of HIV diagnostics is increasingly leaning towards rapid testing solutions like the HIV P24 Rapid Test. As technology advances, these tests are becoming more sophisticated, offering higher accuracy and broader detection capabilities. The future of HIV diagnostics involves integrating rapid tests with digital health systems for better data tracking and patient management. As a cornerstone of early diagnosis, rapid testing will continue to play a vital role in the global fight against HIV, supporting efforts to achieve an HIV-free generation.
Image Description