QL Biotech's Comprehensive and Accurate Mycoplasma Pneumoniae Test
PRINCIPLE
The One Step Rubella(RV) IgG/IgM Test is a qualitative membrane strip based immunoassay for the detection of RV antibodies (IgG and IgM) in Whole Blood /Serum / Plasma. The test device consists of: 1) a burgundy colored conjugate pad containing RV recombinant envelope antigens conjugated with Colloid gold (RV conjugates) and rabbit IgG-gold conjugates,2) a nitrocellulose membrane strip containing two test bands (T1 and T2 bands) and a control band (C band). The T1 band is pre-coated with the antibody for the detection of IgM anti-RV, T2 band is coated with antibody for the detection of IgG anti-RV, and the C band is pre-coated with goat anti rabbit IgG.
When an adequate volume of test specimen is dispensed into the sample well of the test cassette, the specimen migrates by capillary action across the cassette. IgG anti-RV, if present in the
specimen, will bind to the RV conjugates. The immunocomplex is then captured by the reagent pre-coated on the T2 band, forming a burgundy colored T2 band, indicating a RV IgG positive test result and suggesting a recent or repeat infection. IgM anti-RV if present in the specimen will bind to the RV conjugates. The immunocomplex is then captured by the reagent coated on the T1 band, forming a burgundy colored T1 band, indicating a RV IgM positive test result and suggesting a fresh infection. Absence of any T bands (T1 and T2) suggests a negative result. The test contains an internal control (C band) which should exhibit a burgundy colored band of the immunocomplex of goat anti rabbit IgG/rabbit IgG-gold conjugate regardless of the color
development on any of the T bands. Otherwise, the test result is invalid and the specimen must be retested with another device.
Product Detail
-
-
Brand: QL
Specimens: : Whole Blood/Serum/Plasma
Reading time:15 minutes.
Pack:25 T
STORAGE: 2‐30°C
KIT COMPONENTS(Device)
Test devices
Droppers
Buffer
Package insert
-
PRECAUTIONS
- Please read all the information in this package insert before performing the test.
- ● Do not use after the expiration date printed on the foil pouch.
- ● Store in a dry place at 2-30°C or 36-86°F. Do not freeze.
- ● Do not use if pouch is torn or damaged.
- ● Keep out of the reach of children.
- ● For in vitro diagnostic use. Not to be taken internally.
- ● Do not open the test foil pouch until you are ready to start the test.
- ● The used test should be discarded according to local regulations.
TEST RESULTS
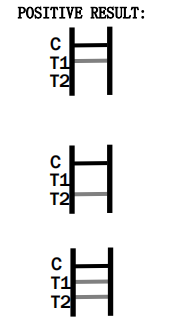
IgM Positive:* The colored line in the control line region (C) appears and a colored line appears in test line region 1 (T1). The result is positive for Rubella virus specific-IgM antibodies and is indicative of primary Rubella infection.
IgG Positive:* The colored line in the control line region (C) appears and a colored line appears in test line region 2 (T2. The result is positive for Rubella virus specific-IgG and is probably indicative of secondary Rubella infection.
IgG and IgM Positive:* The colored line in the control line region (C) appears and two colored lines should appear in test line regions 1 and 2 (T1 and T2). The color intensities of the lines do not have to match.
The result is positive for IgG & IgM antibodies and is indicative of secondary Rubella infection.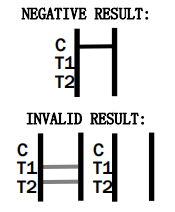
The colored line in the control line region (C) appears. No line appears in test line regions 1 or 2 (T1 or T2).
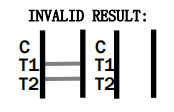
- Control line (C) falls to appear. Insufficient buffer volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the procedure with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
- NOTE:
- The intensity of the color in test region (T) may vary depending on the concentration of aimed substances present in the specimen. Therefore, any shade of color in the test region should be considered positive. Besides, the substances level cannot be determined by this qualitative test.
- Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
Empowering medical professionals with a dependable tool, the Mycoplasma Pneumoniae Test offers quick and precise results, thereby aiding in better diagnostic performance and patient management. Emphasizing quality and effectiveness, this product reflects QL Biotech's staunch dedication to contributing to improved health standards globally. In a world where early and accurate diagnosis can significantly alter medical outcomes, QL Biotech is proud to offer the Mycoplasma Pneumoniae Test, leveraging cutting-edge technology for better, healthier tomorrows. Trust our product for your diagnostic requirements and witness the excellence of QL Biotech's superior biotechnological solutions.


