QL Biotech's Malaria pf Test Kit: Revolutionary HCV Rapid Testing Solution
PRINCIPLE
The HCV Rapid Test Device (Whole Blood /Serum/Plasma) has been designed to detect antibodies to HCV through visual interpretation of color development in the internal strip. The membrane was immobilized with protein A on the test region. During the test, the specimen is allowed to react with colored recombinant HCV antigens colloidal gold conjugates, which were precoated on the sample pad of the test. The mixture then moves on the membrane by a capillary action, and interacts with reagents on the membrane. If there were enough HCV antibodies in specimens, a colored band will from at the test region of the membrane. Presence of this colored band indicates a positive result, while its absence indicates a negative result. Appearance of a colored band at the control region serves as a procedural control. This indicates that proper volume of specimen has been added and membrane wicking has occurred.
Product Detail
Brand: QL
Specimens: : Whole Blood/Serum/Plasma
Reading time:10 minutes.
Pack:25 T
STORAGE: 2‐30°C
KIT COMPONENTS:
Individually packed test strips
Disposable pipettes
Buffer
Package insert
PROCEDURE
- Bring tests, specimens, buffer and/or controls to room temperature (15-30°C) before use.
1. Remove the test from its sealed pouch, and place it on a clean, level surface. Label the device with patient or control identification. To obtain a best result, the assay should be performed within one hour.
2. For Serum or Plasma specimen: Hold the dropper vertically and transfer 2 drops of serum or plasma (approximately 50l ) and 1 drop buffer to the specimen well (S) of the test device, and start the timer.
For Venipuncture Whole Blood specimen: Hold the dropper vertically and transfer 2 drops of
venipuncture whole blood (approximately 50 l) to the specimen well (S) of the test device, then add 2 dropsof buffer and startthe timer.
For FingerstickWhole Bloodspecimen:
To use a capillary tube: Fill the capillary tube and transfer approximately 50l of fingerstick whole blood specimen to the specimen well (S) of the test device, then add 2 drops of buffer
and startthetimer.
To use hanging drop: Allow 2 hanging drop of fingerstick whole blood specimen (approximately 50l ) to fall into the center of the specimen well (S) on the test device, then add 2 dropsof buffer and startthe timer.
3. Wait for the red line(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 20 minutes.
INTERPRETATION OF RESULTS
-
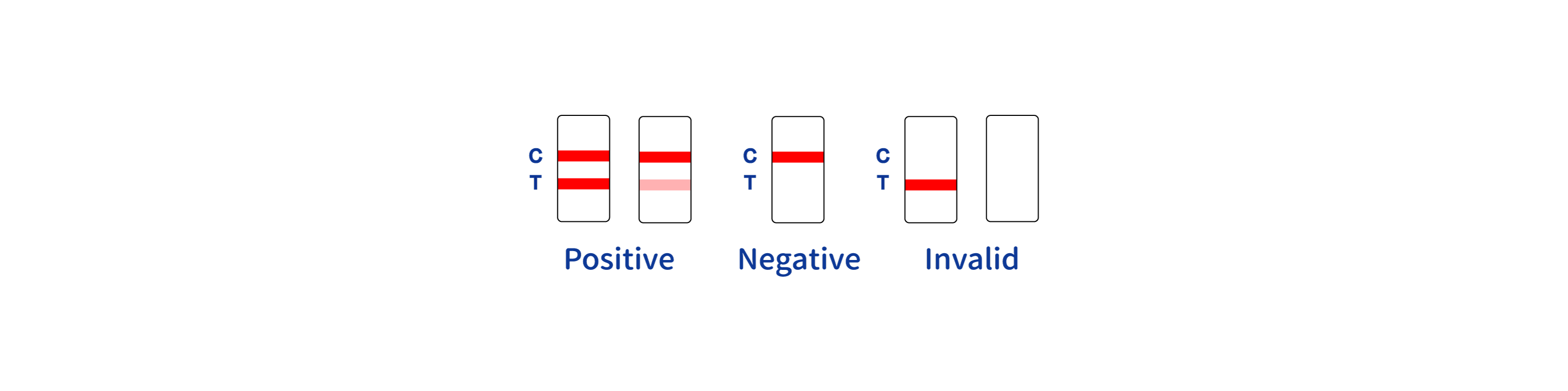
POSITIVERESULT:
* A colored band appears in the control band region (C) and another colored band appears in the T band region.
NEGATIVERESULT:
One colored band appears in the control band region (C). No band appears in the test band region (T).
INVALID RESULT:
Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test.
If the problem persists, discontinue using the kit immediately and contact your local distributor.
NOTE:
1. The intensity of the color in test region (T) may vary depending on the concentration of aimed substances present in the specimen. Therefore, any shade of color in the test region should be considered positive. Besides, the substances level can not be determined by this qualitative test.
2. Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
Our Malaria pf Test Kit is the epitome of our commitment towards facilitating accessible healthcare solutions. We incorporate advanced scientific understanding and innovative technology to create a product that stands out in its effectiveness and reliability. With our HCV rapid test device, we strive to provide you with a convenient, reliable, and quick solution to detect the presence of HCV antibodies, contributing to faster diagnosis and treatment. This product reflects QL Biotech's continual devotion to improving and revolutionizing healthcare through our product offerings. We are dedicated to providing reliable testing solutions that meet everyone's personal and professional needs. QL's Malaria pf Test Kit is one small step towards a world where quick and efficient diagnosis tools are available to all, thereby ensuring a healthier and safer society. Be a part of this revolution, choose our HCV Rapid Test Device for your testing needs today.


