QL Biotech's Rapid HSV-1 Igg/Igm Tests: Pinpointing Typhoid
PRINCIPLE
The Typhoid Ag Rapid Test Device (Feces) is a lateral flow chromatographic immunoassay. The test cassette consists of: 1) a burgundy colored conjugate pad containing S. typhoid antibody conjugated with colloid gold, 2) a nitrocellulose membrane strip containing two test bands (S.typhoid bands) and a control band (C band). The S. typhoid band is pre-coated with monoclonal anti- S. typhoid for the detection of S. typhoid Ag, and the C band is pre-coated with goat anti mouse IgG. When an adequate volume of test specimen is dispensed into the sample well of the cassette, the test specimen migrates by capillary action across the test cassette. S. typhoid Ag if present in the patient specimen will bind to the S. typhoid Ab conjugates. The immunocomplex is then captured on the membrane by the pre-coated S. typhoid antibody, forming a burgundy colored S. typhoid band, indicating a S. typhoid positive test result. Absence of any test bands suggests a negative result. The test contains an internal control (C band) which should exhibit a burgundy colored band of the immunocomplex of goat anti Mouse IgG/Mouse IgG-gold conjugate regardless of the color development on any of the test bands. Otherwise, the test result is invalid and the specimen must be retested with another device.
Product Detail
-
-
-
-
-
-
Brand: QL
Specimens: : Faces
Reading time:10 minutes.
Pack:20 T
STORAGE: 2‐30°C
KIT COMPONENTS
- Test devices
- Droppers
- Single Buffer
- Package insert
-
-
-
-
-
-
ASSAY PROCEDURE
-
Bring tests, specimens, buffer and/or controls to room temperature (15-30°C) before use.
1. Specimen collection and pre-treatment:
1) Unscrew and remove the dilution tube applicator. Be careful not to spill or spatter solution from the tube. Collect specimens by inserting the applicator stick into at least 3 different sites of the feces.
2) Place the applicator back into the tube and screw the cap tightly. Be careful not to break the tip of the dilution tube.
3) Shake the specimen collection tube vigorously to mix the specimen and the extraction buffer. Specimens prepared in the specimen collection tube may be stored for 6 months at -20°C if not tested within 1 hour after preparation.
2. Testing
1) Remove the test from its sealed pouch, and place it on a clean, level surface. Label the test with patient or control identification. To obtain a best result, the assay should be performed within one hour.
2) Using a piece of tissue paper, remove the tip of the dilution tube. Hold the tube vertically and dispense 3 drops of solution into the specimen well (S) of the test device.
Avoid trapping air bubbles in the specimen well (S), and do not drop any solution in observation window.
As the test begins to work, you will see color move across the membrane.
3. Wait for the colored band(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 20 minutes.
INTERPRETATION OF ASSAY RESULT
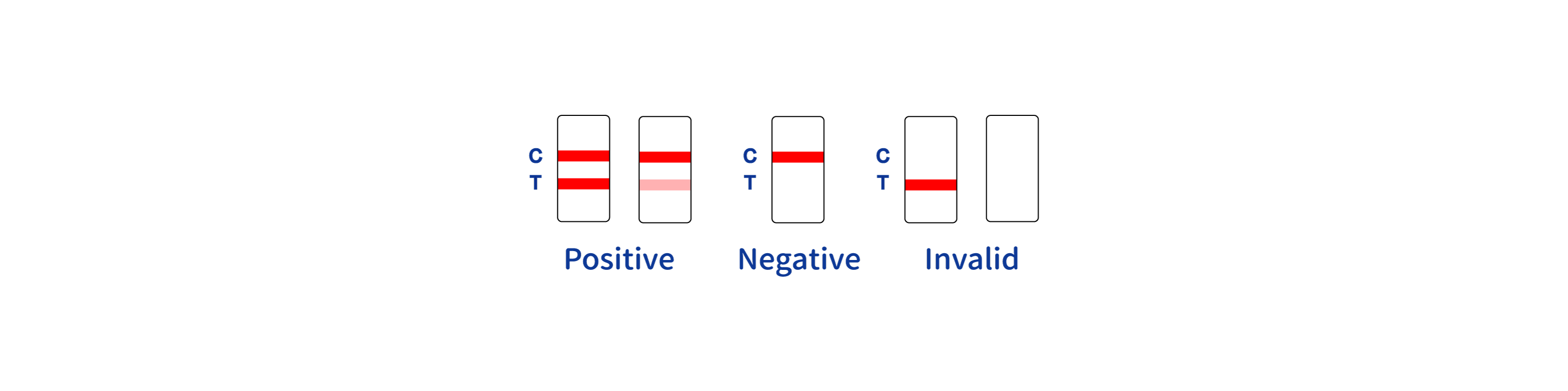
-
-
NOTE:
- 1.The intensity of the color in test region (T) may vary depending on the concentration of aimed substances present in the specimen. Therefore, any shade of color in the test region should be considered positive. Besides, the substances level cannot be determined by this qualitative test.
- 2.Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
Our HSV-1 igg/igm tests stands as a beacon in typhoid testing technology, offering speedy, accurate results that you can trust. The primary component of the test, the lateral flow chromatographic immunoassay, is an advanced scientific technique that harnesses the power of immunochromatography. Our advanced typhoid detection tool boasts a straightforward, user-friendly interface that aids in producing accurate results quickly and efficiently. The Typhoid Ag Rapid Test Device underscores QL Biotech's commitment to leveraging state-of-the-art technology to deliver solutions that address global health challenges. Our focus on the HSV-1 igg/igm tests illustrates our dedication to constantly evolve and enhance our product offerings. This test kit is part of our broader efforts to equip healthcare professionals with the tools they need to diagnose and treat conditions effectively and swiftly. In the high-stakes world of disease identification and management, QL Biotech's Typhoid Ag Rapid Test Device proves to be an indispensable asset. Let our HSV-1 igg/igm tests play a crucial role in your typhoid detection and management strategy today.


