Quick Detection with Cholera O1 Antigen Rapid Test Kit
PRINCIPLE
It is a qualitative membrane based immunoassay for the detection of TP antibodies (IgG and IgM) in whole blood, serum or plasma. In this test procedure, recombinant Syphilis antigen is immobilized in the test line region of the test.
After specimen is added to the specimen well of the device, it reacts with Syphilis antigen coated particles in the test. This mixture migrates chromatographically along the length of the test and interacts with the immobilized Syphilis antigen. The double antigen test format can detect both IgG and IgM in specimens. If the specimen contains TP antibodies, a colored line will appear in the test line region, indicating a positive result. If the specimen does not contain TP antibodies, a colored line will not appear in this region, indicating a negative result. To serve as a procedural control, a colored line will always appear in the control line region, indicating that proper volume of specimen has been added and membrane wicking has occurred.
Product Detail
-
-
-
Brand: QL
1.Specimens: : Serum/Plasma
Reading time:10 minutes.
Pack:25 T
STORAGE: 2‐30°C
KIT COMPONENTS
- Test devices/strips· Droppers
- Buffer · Package insert
Brand: QL
2.Specimens: : Whole Blood/Serum/Plasma
Reading time:10 minutes.
Pack:25 T
STORAGE: 2‐30°C
KIT COMPONENTS
- Test devices/strips· Droppers
- Buffer · Package insert
-
-
DIRECTIONS FOR USE
- 1.Syphilis Rapid Test Device/Strip (Serum/Plasma)
- Allow the test, specimen, buffer and/or controls to reach room temperature (15‐30°C) prior to testing.
- 1.Remove the test strip from the sealed pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
- 2.Hold the dropper vertically and transfer 2 drops of serum or plasma (approximately 50 mL) onto the Specimen Pad of the test strip, then add 1 drop of buffer (approximately 40 mL) and
- start the timer.
- 3.Wait for the colored line(s) to appear. Read results at 10 minutes. Do not interpret the result after 30 minutes.
- 2.Syphilis Rapid Test Device(Whole Blood/Serum/Plasma)
- Allow the test, specimen, buffer and/or controls to reach room temperature (15‐30°C)
- prior to testing.
- 1.Bring the pouch to room temperature before opening it. Remove the test device from the sealed pouch and use it as soon as possible.
- 2.Place the device on a clean and level surface.
- For Serum or Plasma specimen: Hold the dropper vertically and transfer 1 drop of serum or plasma (approximately 25mL) to the specimen well (S), then add 2 drops of buffer (approximately 80mL), and start the timer.
- For Whole Blood specimen: Hold the dropper vertically and transfer 2 drops of whole blood (approximately 80mL) to the specimen well (S), then add 1 drop of buffer (approximately 40 mL), and start the timer.
- 3.Wait for the colored line(s) to appear. Read results at 10 minutes. Do not interpret the result after 30 minutes.
3.Syphilis Rapid Test Strip(Whole Blood/Serum/Plasma)
Allow the test, specimen, buffer and/or controls to reach room temperature (15‐30°C) prior to testing.
1.Remove the test strip from the sealed pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.2.For Serum or Plasma specimens: Hold the dropper vertically and transfer 2 drops of serum or plasma (approximately 50 mL) onto the Specimen Pad of the test strip, then add 1 drop of buffer (approximately 40 mL) and start the timer.
For Venipuncture Whole Blood specimens: Hold the dropper vertically and transfer 2 drops of whole blood (approximately 50 mL) onto the Specimen Pad of the test strip, then add 2 drops of buffer (approximately 80 mL) and start the timer.
For Fingerstick Whole Bloodspecimens:
Fill the capillary tube and transfer approximately 50 mL (or 2 drops) of fingerstick whole blood specimen onto the Specimen Pad of the test strip, then add 2 drops of buffer (approximately 80 mL) and start the timer.
3.Wait for the colored line(s) to appear. Read results at 10 minutes. Do not interpret the result after 30 minutes.INTERPRETATION OF RESULTS
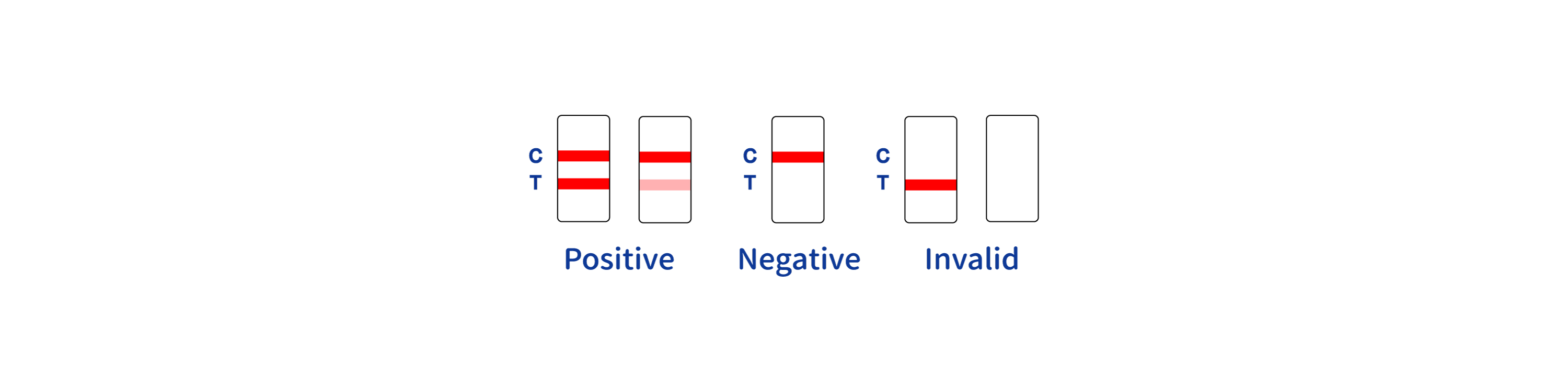
The design of the Cholera O1 Antigen Rapid Test Device/Strip incorporates a qualitative membrane-based immunoassay technology. This enables the detection of cholera O1 antigens with high sensitivity and specificity, ensuring healthcare professionals can make informed decisions quickly. The ease of use is another highlight; the test can be administered with minimal training, producing results in a matter of minutes. This rapid turnaround is crucial for early intervention and controlling the spread of the disease. QL Biotech is committed to advancing public health through innovative diagnostic solutions. Our Cholera O1 Antigen Rapid Test Device/Strip is a testament to this mission, offering a quick, reliable, and accessible tool in the fight against cholera. It's not just a product; it's a lifeline for communities affected by cholera, ensuring that outbreaks can be managed effectively and with the urgency they require.


