Quick & Reliable Malaria PF/PV Rapid Test Kit
PRINCIPLE
The HIV 1/2 Human Immunodeficiency Virus Rapid Test Device (Whole Blood/Serum/Plasma) is a qualitative, membrane based immunoassay for the detection of antibodies to HIV 1/2 in whole blood, serum or plasma. The membrane is pre‐coated with recombinant HIV antigens. During testing, the whole blood, serum or plasma specimen reacts with HIV antigen coated particles in the test strip. The mixture then migrates upward on the membrane chromatographically by capillary action and reacts with recombinant HIV antigen on the membrane in the test line region. If the specimen contains antibodies to HIV 1 and/or HIV 2, a colored line will appear in the test line region indicating a positive result. If the specimen does not contain HIV 1 and/or HIV 2 antibodies, a colored line will not appear in the test line region indicating a negative result. To serve as a procedural control, a colored line will always appear in the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.
Product Detail
Brand: QL
Specimens: : Serum/Plasma
Reading time:10 minutes.
Pack:25 T
STORAGE: 2‐30°C
KIT COMPONENTS(Device)
Test devices
Disposable specimen droppers
Buffer
Package insert
Brand: QL
Specimens: : Whole Blood/Serum/Plasma
Reading time:Strip:15 minutes. Device:10 minutes
Pack:25 T
STORAGE: 2‐30°C
KIT COMPONENTS(Device)
Test devices
Disposable specimen droppers
Buffer
Package insert
KIT COMPONENTS(Strip)
Test strips
Disposable specimen droppers
Buffer
Package insert
Test cards
DIRECTIONS FOR USE
-
HIV 1/2 Rapid Test Strip(Whole Blood/Serum/Plasma)
Allow the test strip, specimen, buffer, and/or controls to equilibrate to room temperature (15‐30°C) prior to testing.
1. Remove the test strip from the foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
2. Peel off the tape from the test card, and stick the test strip in the middle of test card with arrows pointing down on the test card.
For Serum, Plasma or Venipuncture Whole Blood specimen: Hold the dropper vertically and transfer 2 drops of serum, plasma or venipuncture whole blood (approximately 80 L) to the
“Specimen Pad” of the test strip, then add 1 drop of buffer (approximately 40L) and start the timer.
For Fingerstick Whole Blood specimen:
To use a capillary tube: Fill the capillary tube and transfer approximately 80L of fingerstick whole blood specimen to the “Specimen Pad” of the test strip, then add 1 drop of buffer (approximately 40L) and start the timer.
To use hanging drop: Allow 2 hanging drops of fingerstick whole blood specimen to fall into the center of the “Specimen Pad” on the test strip, then add 1 drop of buffer (approximately
40L) and start the timer.
3. Wait for the colored line(s) to appear. The result should be read at 15 minutes. Do not interpret results after 20 minutes.
HIV 1/2 Rapid Test Strip(Serum/Plasma)
HIV 1/2 Rapid Test Device (Whole Blood/Serum/Plasma)
Allow the test device, specimen, buffer, and/or controls to equilibrate to room temperature
(15‐30°C) prior to testing.
1. Remove the test device from the foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
2. Place the test device on a clean and level surface. Hold the dropper vertically and transfer 2 drops of serum, plasma (approximately 80L) to the specimen well (S) of the sample pad then add 1 drop of buffer (approximately 40L) and start thetimer.
3. Wait for the colored line(s) to appear. The result should be read at 10 minutes. Do not interpret results after 20 minutes.
HIV 1/2 Rapid Test Device(Serum/Plasma)
Allow the test device, specimen, buffer, and/or controls to equilibrate to room temperature (15‐30°C) prior to testing.
1. Remove the test device from the foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
2. Place the test device on a clean and level surface.
For Serum, Plasma specimen: Hold the dropper vertically and transfer 2 drops of serum, plasma (approximately 80L) to the specimen well (S) of the test device, then add 1 drop of buffer (approximately 40L) and start the timer.
3. Wait for the colored line(s) to appear. The result should be read at 10 minutes. Do not interpret results after 20 minutes.
INTERPRETATION OF RESULTS
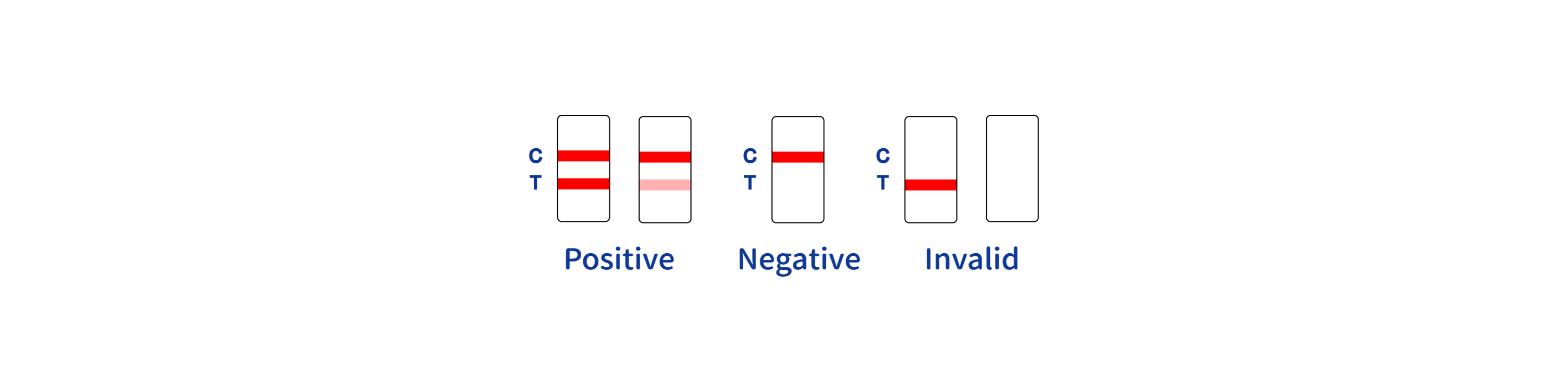
INVALIDRESULT:
Control line (C) falls to appear. Insufficient buffer volume or incorrect procedural techniques are the most likely reasons for control line failure.
Review the procedure and repeat the procedure with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
*NOTE: The intensity of the color in the test line region (T) will vary depending on the concentration of HIV antibodies present in the specimen. Therefore, any shade of colored in the test region (T) should be considered positive.
Our Malaria PF/PV Rapid Test Kit is designed for simplicity and accuracy, ensuring that health professionals and individuals alike can trust in the results. Utilizing a state-of-the-art, membrane-based immunoassay, our test detects the presence of malaria antigens in whole blood, serum, or plasma samples. The principle of the test rests on the recognition of specific malaria antigens by antibodies immobilized on a test strip, providing results that are both rapid and sensitive. The necessity of early and accurate malaria diagnosis cannot be overstated. Malaria, a disease transmitted through the bite of an infected Anopheles mosquito, poses a significant threat to global health, particularly in tropical and subtropical regions. Early detection and treatment are crucial to preventing severe illness and limiting the spread of the disease. Our Malaria PF/PV Rapid Test Kit offers a solution to this challenge, providing results in as little as 15 minutes. This rapid turnaround time ensures that individuals receive the prompt care and treatment needed to combat the disease effectively.


