Rapid Gonorrhea Test Cassette by QL Biotech: Filariasis IgG/IgM Detection
Product Detail
Brand: QL
Specimens: : Whole Blood/Serum/Plasma
Reading time: in 15 minutes.
Pack:25 T
STORAGE 2‐30°C
KIT COMPONENTS(Device)
Individually packed test devices
Each device contains a strip with colored conjugates
and reactive reagents pre-spreaded at the
corresponding regions
Disposable pipettes
For adding specimens use
Buffer
Phosphate buffered saline and preservative
Package insert
For operation instruction
SUMMARY AND EXPLANATION OF THE TEST
The lymphatic filariasis known as Elephantiasis, mainly caused by W. bancrofti and B. malayi, affects about 120 million people over 80 countries1,2. The disease is transmitted to humans by
the bites of infected mosquitoes within which the microflariae sucked from an infected human subject develops into third-stage larvae. Generally, repeated and prolonged exposure to infected larvae is required for establishment of human infection.
The definitive parasitologic diagnosis is the demonstration of microflariae in blood samples3.
However, this gold standard test is restricted by the requirement for nocturnal blood collection and lack of adequate sensitivity. Detection of circulating antigens is commercially available. Its
usefulness is limited for W. bancrofti4. In addition, microfilaremia and antigenemia develop from months to years after exposure.
Antibody detection provides an early means to detect filarial parasite infection. Presence of IgM to the parasite antigens suggest current infection, whereas, IgG corresponds to late stage of infection or past infection5. Furthermore, identification of conserved antigens allows ‘pan-filaria’ test to be applicable. Utilization of recombinant proteins eliminates cross-reaction with
individuals having other parasitic diseases6. The Filariasis IgG/IgM Rapid Test uses conserved recombinant antigens to simultaneously detect IgG and IgM to the W. bancrofti and B. malayi parasites without the restriction on specimen collection.
ASSAY PROCEDURE
For whole blood test
Apply 1 drop of whole blood (about 40-50 µL) into the sample well. Then add 1 drop (about 35-50 µL) of Sample Diluent immediately.
For serum or plasma test
Fill the pipette dropper with the specimen. Holding the dropper vertically, dispense 1 drop (about 30-45 µL) of specimen into the sample well making sure that there are no air bubbles.
Then add 1 drop (about 35-50 µL) of Sample Diluent immediately.
Set up timer. Results can be read in 15 minutes. Positive results can be visible in as short as 1 minute. Don’t read result after 15 minutes.INTERPRETATION OF ASSAY RESULT
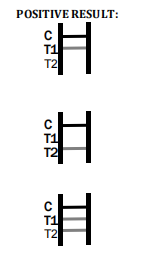
1.In addition to the presence of C band, if only T1 band is developed, the test indicates for the presence of anti-W. bancrofti or B. malayi IgG antibody. The result is positive.
2.In addition to the presence of C band, if only T2 band is developed, the test indicates for the presence of anti-W. bancrofti or B. malayi IgM antibody. The result is positive.
3.In addition to the presence of C band, both T1 and T2 bands are developed, the test indicates for the presence of both IgG and IgM anti-W. bancrofti or B. malayi. The result is also positive.
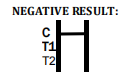
If only the C band is present, the absence of any burgundy color in the both test bands (T1 and T2) indicates that no anti-W. bancrofti or -B. malayi antibody is detected in the specimen. The
result is negative.
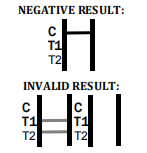
If no C band is developed, the assay is invalid regardless of any burgundy color in the test bands. Repeat the assay with a new device.
QL Biotech is a leading player in the biotech industry, known for integrating innovative technologies into its products. The Gonorrhea Test Cassette is a prime example of this commitment to innovation. It is a powerful tool in the hands of medical professionals, enabling them to diagnose Filariasis effectively and efficiently. In conclusion, the Gonorrhea Test Cassette from QL Biotech simplifies the diagnostic process, making it swift and accurate. It's time to step up your medical practice with this Filariasis IgG/IgM Rapid Test Device and experience quality, efficiency, and reliability like never before. Embrace the future of biotech with QL.


