Rapid HIV Detection Kit – Mycoplasma Pneumoniae Test Optimized
PRINCIPLE
Human Immunodeficiency Virus (HIV) Ab & P24 Ag Rapid Test Device (Whole Blood/Serum/Plasma) is a qualitative, membrane based immunoassay for the detection of antibodies to HIV 1&2 and P24 antigen in whole blood, serum or plasma. The membrane is precoated with recombinant HIV antigens to HIV 1&2 antibody and P24 antibody to HIV P24 antigen. During testing, the whole blood, serum or plasma specimen reacts with HIV antigen or P24 antibody coated particles in the test strip. The mixture then migrates upward on the membrane chromatographically by capillary action and reacts with recombinant HIV antigen or P24 antibody on the membrane in the test line regions. If the specimen contains antibodies to HIV 1 and/or HIV 2 and/or P24 antigen, a colored line will appear in the test line region indicating a positive result. If the specimen does not contain HIV 1 and/or HIV 2 antibodies and/or P24 antigen, no colored line will appear in the test line region indicating a negative result. To serve as a procedural control, a colored line will always appear in the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.
MATERIALS
Materials Provided
● Test devices ● Disposable specimen droppers
● Buffer ● Package insert
Materials Required But Not Provided
● Specimen collection containers ● Lancets (for fingerstick whole blood only)
● Centrifuge (for plasma only) ● Timer
● Disposable heparinized capillary tubes and dispensing bulb (for fingerstick whole blood only)
DIRECTIONS FOR USE
-
● Test devices
● Disposable specimen droppers
● Buffer
● Package insert
Materials Required But Not Provided
● Specimen collection containers
● Lancets (for fingerstick whole blood only)
● Centrifuge (for plasma only)
● Timer
● Disposable heparinized capillary tubes and dispensing bulb (for fingerstick whole blood only)
PERFORMANCE CHARACTERISTICS
Sensitivity
Human Immunodeficiency Virus (HIV) Ab & P24 Ag Rapid Test Device (Whole
Blood/Serum/Plasma) has been tested by anti-HIV 1 low titer performance panel, anti-HIV 2 performance panel and anti-HIV 1 seroconversion panel (Boston Biomedica, Inc.). And it has also been compared with leading commercial EIA HIV test on clinical specimens. The results show that
HIV 1&2 Human Immunodeficiency Virus Rapid Test Device (Whole Blood/Serum/Plasma) is very sensitive to HIV 1 and/or HIV 2 antibodies.
Specificity
The specificity of the test is comparable to a leading commercial HIV EIA test. Human Immunodeficiency Virus (HIV) Ab & P24 Ag Rapid Test Device (Whole Blood/Serum/Plasma) is highly specific for anti-HIV 1 and/or HIV 2 compared to a leading commercial HIV EIA test.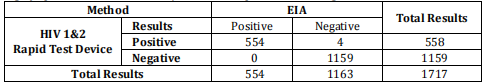
Relative Sensitivity: 99.9% (99.3%-100.0%)*Relative Specificity: 99.6% (99.1%-99.9%)*
Relative Accuracy: 99.8% (99.4%-99.9%)* * 95% Confidence Interval
Precision
Intra Assay
Within-run precision has been determined by using 15 replicates of three specimens: a negative, a low positive and a high positive. The negative, low positive and high positive values were correctly identified 99.5% of the time.
Inter-Assay
Between-run precision has been determined by 15 independent assays on the same three specimens: a negative, a low positive and a high positive. Three different lots of Human
Immunodeficiency Virus (HIV) Ab & P24 Ag Rapid Test Device (Whole Blood/Serum/Plasma) have been tested using negative, low positive and high positive specimens. The specimens were correctly identified 99.5% of the time.
The cornerstone of this device lies in its sophisticated, membrane-based immunoassay technology. This technique is finely tuned to detect antibodies against both HIV-1 and HIV-2, along with the P24 antigen, offering an unparalleled breadth of viral detection from a single sample. But our commitment to advancing healthcare solutions doesn't stop there. Recognizing the increasing incidence of Mycoplasma Pneumoniae infections and their clinical relevance, especially in compromised patients, we've seamlessly integrated a testing component for this pathogen, echoing our dedication to comprehensive patient care and diagnostic accuracy. Utilizing a straightforward, user-friendly design, our Rapid Test Device ensures healthcare professionals can deliver prompt and reliable results. Its intuitive operation streamlines the testing process, reducing the window for pre-diagnostic anxiety and enabling faster initiation of appropriate therapeutic interventions. The device's sensitivity and specificity have been meticulously calibrated to meet the demanding standards of clinical diagnostics, ensuring each test conducted contributes towards accurate epidemiological data and, ultimately, towards the global fight against HIV and Mycoplasma Pneumoniae co-infections. In doing so, QL Biotech not only stands at the forefront of diagnostic innovations but also reinforces its commitment to public health and the well-being of communities worldwide.


