Rapid HIV Detection: Ns1/Igm/Igg Combotest Panel
PRINCIPLE
Human Immunodeficiency Virus (HIV) Ab & P24 Ag Rapid Test Device (Whole Blood/Serum/Plasma) is a qualitative, membrane based immunoassay for the detection of antibodies to HIV 1&2 and P24 antigen in whole blood, serum or plasma. The membrane is precoated with recombinant HIV antigens to HIV 1&2 antibody and P24 antibody to HIV P24 antigen. During testing, the whole blood, serum or plasma specimen reacts with HIV antigen or P24 antibody coated particles in the test strip. The mixture then migrates upward on the membrane chromatographically by capillary action and reacts with recombinant HIV antigen or P24 antibody on the membrane in the test line regions. If the specimen contains antibodies to HIV 1 and/or HIV 2 and/or P24 antigen, a colored line will appear in the test line region indicating a positive result. If the specimen does not contain HIV 1 and/or HIV 2 antibodies and/or P24 antigen, no colored line will appear in the test line region indicating a negative result. To serve as a procedural control, a colored line will always appear in the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.
MATERIALS
Materials Provided
● Test devices ● Disposable specimen droppers
● Buffer ● Package insert
Materials Required But Not Provided
● Specimen collection containers ● Lancets (for fingerstick whole blood only)
● Centrifuge (for plasma only) ● Timer
● Disposable heparinized capillary tubes and dispensing bulb (for fingerstick whole blood only)
DIRECTIONS FOR USE
-
● Test devices
● Disposable specimen droppers
● Buffer
● Package insert
Materials Required But Not Provided
● Specimen collection containers
● Lancets (for fingerstick whole blood only)
● Centrifuge (for plasma only)
● Timer
● Disposable heparinized capillary tubes and dispensing bulb (for fingerstick whole blood only)
PERFORMANCE CHARACTERISTICS
Sensitivity
Human Immunodeficiency Virus (HIV) Ab & P24 Ag Rapid Test Device (Whole
Blood/Serum/Plasma) has been tested by anti-HIV 1 low titer performance panel, anti-HIV 2 performance panel and anti-HIV 1 seroconversion panel (Boston Biomedica, Inc.). And it has also been compared with leading commercial EIA HIV test on clinical specimens. The results show that
HIV 1&2 Human Immunodeficiency Virus Rapid Test Device (Whole Blood/Serum/Plasma) is very sensitive to HIV 1 and/or HIV 2 antibodies.
Specificity
The specificity of the test is comparable to a leading commercial HIV EIA test. Human Immunodeficiency Virus (HIV) Ab & P24 Ag Rapid Test Device (Whole Blood/Serum/Plasma) is highly specific for anti-HIV 1 and/or HIV 2 compared to a leading commercial HIV EIA test.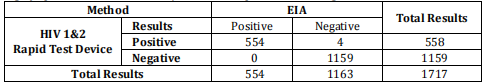
Relative Sensitivity: 99.9% (99.3%-100.0%)*Relative Specificity: 99.6% (99.1%-99.9%)*
Relative Accuracy: 99.8% (99.4%-99.9%)* * 95% Confidence Interval
Precision
Intra Assay
Within-run precision has been determined by using 15 replicates of three specimens: a negative, a low positive and a high positive. The negative, low positive and high positive values were correctly identified 99.5% of the time.
Inter-Assay
Between-run precision has been determined by 15 independent assays on the same three specimens: a negative, a low positive and a high positive. Three different lots of Human
Immunodeficiency Virus (HIV) Ab & P24 Ag Rapid Test Device (Whole Blood/Serum/Plasma) have been tested using negative, low positive and high positive specimens. The specimens were correctly identified 99.5% of the time.
Our test leverages a state-of-the-art, membrane-based immunoassay, which is designed to streamline the testing process while ensuring the utmost accuracy and reliability. The core of this device's effectiveness lies in its ability to simultaneously detect the early markers of HIV - including the critical P24 antigen, often present before antibodies are detectable - alongside the traditional antibody test. This dual-action capability ensures that individuals can receive earlier diagnosis and subsequent care, which can be crucial for managing the virus effectively. Furthermore, the inclusion of the Ns1/Igm/Igg Combotest Panel extends the device's capabilities, providing a comprehensive overview of a person's serological status with respect to HIV. This panel facilitates the differentiation between acute and later-stage infections, offering insights that are critical for clinicians to tailor individual treatment plans. By integrating this panel, our device not only caters to the urgent need for rapid HIV testing but also embodies QL Biotech's commitment to pioneering advancements in the medical diagnostic field, helping healthcare providers offer prompt and accurate care.


