Reliable Herpes (HSV-1, HSV-2) Test Kits - Dengue Test Cassette
PRINCIPLE
HSV‐1 IgG/IgM (HSV-2 IgG/IgM)Test is a qualitative membrane strip based immunoassay for the detection of HSV‐1 antibodies (IgG and IgM) in Whole Blood/Serum/Plasma. The test device consists of: 1) a burgundy colored conjugate pad containing HSV recombinant envelope antigens conjugated with Colloid gold (HSV conjugates) and rabbit IgG‐gold conjugates,2) a nitrocellulose membrane strip containing two test bands (T1 and T2 bands) and a control band (C band). The T1 band is pre‐coated with the antibody for the detection of IgM anti‐HSV, T2 band is coated with antibody for the detection of IgG anti‐HSV, and the C band is pre‐coated with goat anti rabbit IgG.
When an adequate volume of test specimen is dispensed into the sample well of the test cassette, the specimen migrates by capillary action across the cassette. IgG anti‐HSV, if present in the specimen, will bind to the HSV conjugates. The immunocomplex is then captured by the reagent pre‐coated on the T2 band, forming a burgundy colored T2 band, indicating a HSV IgG positive test result and suggesting a recent or repeat infection. IgM anti‐HSV if present in the specimen will bind to the HSV conjugates. The immunocomplex is then captured by the reagent coated on the T1 band, forming a burgundy colored T1 band, indicating a HSV IgM positive test result and suggesting a fresh infection. Absence of any T bands (T1 and T2) suggests a negative result. The test contains an internal control (C band) which should exhibit a burgundy colored band of the immunocomplex of goat anti rabbit IgG/rabbit IgG‐gold conjugate regardless of the color development on any of the T bands. Otherwise, the test result is invalid and the specimen must be retested with another device.
Product Detail
Brand: QL
Specimens: : Whole Blood/Serum/Plasma
Reading time:15 minutes.
Pack:25 T
STORAGE: 2‐30°C
KIT COMPONENTS(Device)
Individually packed test devices
Disposable pipettes
Buffer
Package insert
PROCEDURE
- Allow the test, specimen, buffer and/or controls to reach room temperature 15-30℃ (59-86℉) prior to testing.
1. Bring the pouch to room temperature before opening it. Remove the test device from the sealed pouch and use it as soon as possible.
2. Place the test device on a clean and level surface.
3. Hold the dropper vertically and transfer 1 drop of specimen (approximately 10μl) to the specimen well(S) of the test device, then add 2 drops of buffer (approximately 70μl) and start the timer. See illustration below.
4. Wait for the colored line(s) to appear. Read results at 15 minutes. Do not interpret the result after 20 minutes.
Notes:
Applying sufficient amount of specimen is essential for a valid test result. If migration (the wetting of membrane) is not observed in the test window after one minute, add one more drop of buffer to the specimen well.
INTERPRETATION OF RESULTS
-
POSITIVE RESULT:
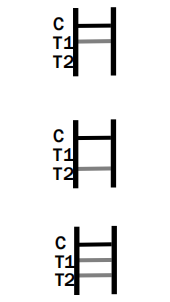
IgM Positive:* The colored line in the control line region (C) appears and a colored line appears in test line region 1 (T1). The result is positive for HSV virus specific-IgM antibodies and is indicative of primary HSV infection.
IgG Positive:* The colored line in the control line region (C) appears and a colored line appears in test line region 2 (T2). The result is positive for HSV virus specific-IgG and is probably indicative of secondary HSV infection.
IgM and IgG Positive:* The colored line in the control line region (C) appears and two colored lines should appear in test line regions 1 and 2 (T1 and T2). The color intensities of the lines do not have to match.
The result is positive for IgM & IgG antibodies and is indicative of secondary HSV infection.
NEGATIVE RESULT: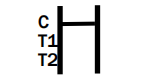
The colored line in the control line region (C) appears. No line appears in test line regions 1 or 2 (T1 or T2).
INVALID RESULT: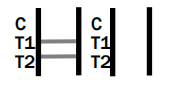
Control line (C) falls to appear. Insufficient buffer volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the procedure with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
NOTE:
1. The intensity of the color in test region (T) may vary depending on the concentration of aimed substances present in the specimen. Therefore, any shade of color in the test region
should be considered positive. Besides, the substances level cannot be determined by this qualitative test.
2. Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
Our HSV-1 IgG/IgM and HSV-2 IgG/IgM Test Devices are meticulously designed for the qualitative detection of antibodies (IgG and IgM) specific to the Herpes Simplex Virus in human Whole Blood, Serum, or Plasma. Utilizing a premium quality membrane strip based immunoassay, these kits ensure that healthcare professionals can obtain accurate and reliable results within a minimal timeframe. The importance of a swift diagnosis cannot be overstated, particularly when it comes to managing and treating viral infections effectively. The core principle of our test kits revolves around the immunoassay technique, a proven method that has been the cornerstone of infectious disease detection for decades. By targeting HSV-1 and HSV-2 antibodies, our test devices provide a comprehensive tool for assessing exposure to the herpes simplex virus, which is crucial for prompt treatment and management. This innovative approach not only elevates the standard of diagnostic testing but also embodies our dedication to enhancing patient care through advanced biotechnological solutions.


