Reliable Supplier of Rapid Test Flu A B Devices
Product Main Parameters
| Parameter | Details |
|---|---|
| Brand | QL |
| Certificate | CE / TGA / ISO13485 |
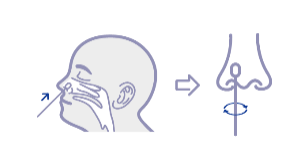
| Specimen | Nasal swab |
| Pack | 1 test, 5 tests, 20 tests |
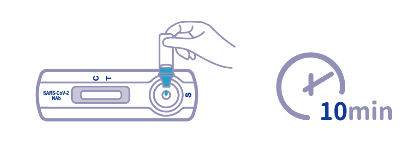
| Reading Time | 10 Minutes |
Common Product Specifications
| Contents | Details |
|---|---|
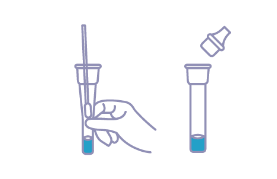
| Contents | Cassette, Sterile Nasal Swab, Buffer, Package Insert |
| Storage | 2-30℃ |
| Shelf Life | 2 Years |
Product Manufacturing Process
The Rapid Test Flu A/B harnesses immunoassay technology for the detection of influenza antigens. This involves coating a nitrocellulose membrane with anti-influenza antibodies that capture antigens present in the specimen if present. A colloidal gold or latex microsphere-conjugated secondary antibody labels these immune complexes, producing visible colored bands. This straightforward yet precise technology aligns with current practices for antigen detection, offering specificity and sensitivity essential for clinical settings. Continuous advancements focus on enhancing assay sensitivity, reducing false negatives, and increasing the reliability of rapid tests. Such innovations ensure a high level of confidence in results, crucial for patient management and outbreak control.
Product Application Scenarios
Rapid Test Flu A/B devices are essential in diverse healthcare environments like clinics, hospitals, and emergency departments, where immediate influenza detection is critical for patient care. Their implementation is particularly valuable during flu seasons when prompt identification of Influenza A and B allows for timely antiviral treatment and infection control. Such applications are supported by studies emphasizing rapid testing's role in reducing influenza transmission, hospital admissions, and enhancing patient outcomes. The tests' ease of use and quick results make them suitable for point-of-care settings, effectively bridging the gap between clinical suspicion and laboratory confirmation.
Product After-Sales Service
Our comprehensive after-sales support ensures satisfaction with every purchase. We provide technical assistance, and user guidance, and address any inquiries regarding the Rapid Test Flu A/B devices. Our dedicated team is readily available to facilitate training sessions and troubleshoot as needed, ensuring seamless integration into your practice. Moreover, we offer a warranty and easy return process for any faulty products, reaffirming our commitment to quality and reliability.
Product Transportation
We ensure the safe and timely delivery of Rapid Test Flu A/B devices through robust packaging and reliable logistics partnerships. Our products are stored at optimal conditions and dispatched with care to maintain their integrity during transit. Clients receive tracking details for real-time updates, guaranteeing peace of mind from dispatch to delivery.
Product Advantages
- Fast results within 10 minutes.
- Simple procedure with minimal training required.
- High specificity and reliable sensitivity.
- Convenient for various healthcare settings.
- Certified and compliant with international standards.
Product FAQ
- How does the Rapid Test Flu A/B supplier device work? The device detects influenza antigens using immunoassay technology. A positive result is indicated by visible colored bands, reflecting the presence of Influenza A or B.
- What is the reading time for results? Results are available within 10 minutes, allowing for quick decision-making in clinical settings.
- What certifications does the supplier provide? The Rapid Test Flu A/B device is CE/TGA/ISO13485 certified, ensuring compliance with international quality standards.
- Can this test be used for self-testing? While designed for professional use, the test's simplicity makes it feasible for guided self-testing under supervision.
- What is the shelf life of the device? The test device boasts a 2-year shelf life, provided it is stored appropriately.
- Is it necessary to follow up on negative results? Due to the lower sensitivity compared to RT-PCR, negative results may require confirmation with another method, especially in symptomatic patients.
- How should the test components be disposed of? All used components must be disposed of as biohazard waste to prevent contamination.
- Can the test differentiate between active and past infections? The test detects antigens present during an active infection but may not differentiate from those of a resolved infection.
- In what settings is this test most useful? Ideal for emergency rooms, outpatient clinics, and other point-of-care settings, offering immediate influenza detection for timely intervention.
- What support does the supplier offer post-purchase? We provide customer service for troubleshooting, training, and warranty claims, ensuring a smooth user experience.
Product Hot Topics
- The Evolution of Rapid Test Flu A/B Technology: A Supplier's Perspective As a supplier of Rapid Test Flu A/B devices, we've witnessed significant advancements in testing technology. While early rapid tests offered convenience, they often struggled with sensitivity issues. Modern innovations focus on enhancing detection capabilities, minimizing false negatives and improving overall test reliability. Suppliers play a crucial role in integrating these advancements into practical solutions for healthcare providers, ensuring that rapid tests continue to meet evolving clinical needs and quality standards.
- The Role of Rapid Test Flu A/B in Managing Flu Outbreaks Suppliers of the Rapid Test Flu A/B have seen firsthand the impact these devices have during influenza outbreaks. Quick and accurate diagnosis is vital for managing patient flow, initiating timely treatments, and implementing infection control measures. By providing reliable testing solutions, suppliers help healthcare facilities maintain high standards of care even under strain, ultimately reducing the burden of flu seasons.
- Quality Assurance in Rapid Test Flu A/B Production: Insights from a Supplier As a supplier, ensuring the quality of Rapid Test Flu A/B devices is paramount. This involves stringent manufacturing processes, rigorous quality control checks, and adherence to international standards like CE/TGA/ISO13485. Through these measures, suppliers guarantee that healthcare providers receive products that perform consistently, supporting accurate and effective patient care.
- Meeting Diverse Clinical Needs: The Versatility of Rapid Test Flu A/B The Rapid Test Flu A/B's utility across various healthcare environments underscores its importance as a diagnostic tool. Suppliers understand the need for versatile solutions capable of adapting to different clinical scenarios, from large hospitals to remote clinics. By offering customizable testing options, suppliers ensure that every healthcare provider can access the diagnostic tools necessary to manage flu effectively.
- Innovations in Rapid Test Flu A/B: What Suppliers are Bringing to the Table Suppliers play a critical role in advancing Rapid Test Flu A/B technologies, driving innovation to meet clinical demands. From enhanced sensitivity to faster processing times, ongoing innovation efforts ensure that these tests remain at the forefront of diagnostic capabilities. Suppliers continuously collaborate with researchers and healthcare professionals to refine test designs, ultimately supporting better clinical outcomes.
- The Importance of Supplier Support in Rapid Test Flu A/B Implementation Effective supplier support is integral to the successful adoption of Rapid Test Flu A/B devices. Suppliers provide essential training, troubleshooting, and guidance to healthcare providers, ensuring seamless integration into clinical workflows. This support not only helps overcome operational challenges but also enhances user confidence in the testing process, leading to more consistent and reliable test results.
- Adapting Rapid Test Flu A/B to Post-Pandemic Healthcare Landscapes In the post-pandemic era, the role of Rapid Test Flu A/B suppliers has evolved. As healthcare systems adapt to new challenges, suppliers are key in providing diagnostic tools that align with updated protocols. This adaptability ensures that rapid testing continues to be a viable option for influenza management, even as healthcare priorities shift.
- The Ethical Considerations of Rapid Test Flu A/B Distribution by Suppliers Suppliers of Rapid Test Flu A/B devices must navigate complex ethical considerations, balancing accessibility, affordability, and quality. Ensuring equitable distribution while maintaining high standards of product performance requires a nuanced approach, guided by ethical principles and a commitment to public health.
- Navigating Regulatory Challenges as a Rapid Test Flu A/B Supplier Regulatory compliance is a cornerstone of responsible Rapid Test Flu A/B supply. Suppliers must stay abreast of changing regulations and standards, ensuring that their products meet all necessary criteria. This vigilance not only supports market access but also upholds the reputation and integrity of the supplier's brand.
- Customer Feedback: A Supplier's Guide to Improving Rapid Test Flu A/B Customer feedback is invaluable to suppliers of Rapid Test Flu A/B devices, providing insights into user experiences and areas for improvement. By actively seeking and incorporating client feedback, suppliers can enhance product features, optimize performance, and better meet the needs of healthcare providers, ultimately leading to improved patient care outcomes.
Image Description