Reliable Tuberculosis Test Strip for Rapid HCV Detection
PRINCIPLE
The HCV Rapid Test Device (Whole Blood /Serum/Plasma) has been designed to detect antibodies to HCV through visual interpretation of color development in the internal strip. The membrane was immobilized with protein A on the test region. During the test, the specimen is allowed to react with colored recombinant HCV antigens colloidal gold conjugates, which were precoated on the sample pad of the test. The mixture then moves on the membrane by a capillary action, and interacts with reagents on the membrane. If there were enough HCV antibodies in specimens, a colored band will from at the test region of the membrane. Presence of this colored band indicates a positive result, while its absence indicates a negative result. Appearance of a colored band at the control region serves as a procedural control. This indicates that proper volume of specimen has been added and membrane wicking has occurred.
Product Detail
Brand: QL
Specimens: : Whole Blood/Serum/Plasma
Reading time:10 minutes.
Pack:25 T
STORAGE: 2‐30°C
KIT COMPONENTS:
Individually packed test strips
Disposable pipettes
Buffer
Package insert
PROCEDURE
- Bring tests, specimens, buffer and/or controls to room temperature (15-30°C) before use.
1. Remove the test from its sealed pouch, and place it on a clean, level surface. Label the device with patient or control identification. To obtain a best result, the assay should be performed within one hour.
2. For Serum or Plasma specimen: Hold the dropper vertically and transfer 2 drops of serum or plasma (approximately 50l ) and 1 drop buffer to the specimen well (S) of the test device, and start the timer.
For Venipuncture Whole Blood specimen: Hold the dropper vertically and transfer 2 drops of
venipuncture whole blood (approximately 50 l) to the specimen well (S) of the test device, then add 2 dropsof buffer and startthe timer.
For FingerstickWhole Bloodspecimen:
To use a capillary tube: Fill the capillary tube and transfer approximately 50l of fingerstick whole blood specimen to the specimen well (S) of the test device, then add 2 drops of buffer
and startthetimer.
To use hanging drop: Allow 2 hanging drop of fingerstick whole blood specimen (approximately 50l ) to fall into the center of the specimen well (S) on the test device, then add 2 dropsof buffer and startthe timer.
3. Wait for the red line(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 20 minutes.
INTERPRETATION OF RESULTS
-
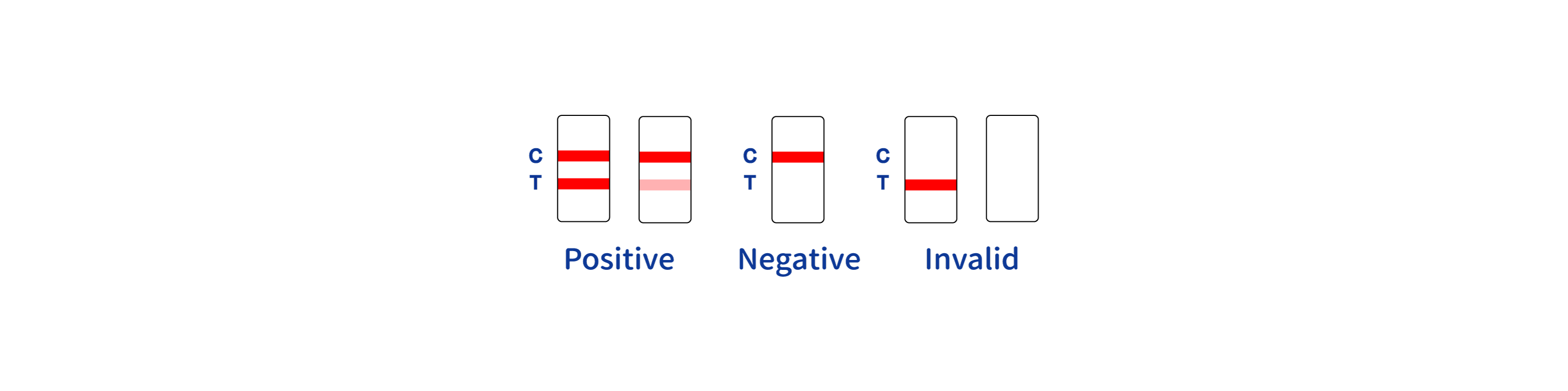
POSITIVERESULT:
* A colored band appears in the control band region (C) and another colored band appears in the T band region.
NEGATIVERESULT:
One colored band appears in the control band region (C). No band appears in the test band region (T).
INVALID RESULT:
Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test.
If the problem persists, discontinue using the kit immediately and contact your local distributor.
NOTE:
1. The intensity of the color in test region (T) may vary depending on the concentration of aimed substances present in the specimen. Therefore, any shade of color in the test region should be considered positive. Besides, the substances level can not be determined by this qualitative test.
2. Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
Understanding the immense impact that early and accurate detection of HCV can have on treatment outcomes, QL Biotech has committed itself to research and innovation, ensuring the HCV Rapid Test Device exceeds the highest standards of diagnostic performance. With its proven high sensitivity and specificity, the device offers peace of mind in just minutes. Our commitment to reliability and accessibility does not end with the device itself. QL Biotech also provides comprehensive support and education, empowering users to conduct tests with confidence and understand their results correctly. Whether for routine screenings, pre-donation testing, or surveillance in areas with high prevalence rates, the HCV Rapid Test Device stands as a beacon of hope and a testament to the power of modern medical technology. In conclusion, as the global healthcare landscape continues to evolve, so too does the need for diagnostic solutions that are not only accurate but also accessible and easy to use. QL Biotech’s HCV Rapid Test Device, inspired by the principles of rapid detection and simplicity, answers this call. It offers a reliable, user-friendly, and efficient means of screening for HCV, underpinned by the company's dedication to quality, innovation, and the well-being of individuals worldwide.


