Rota/Adeno Combo Test: Rapid Detection for Efficient Diagnosis
PRINCIPLE
The Strep A Rapid Test Device/strip is a qualitative, lateral flow immunoassay for the detection of Strep A carbohydrate antigen in a throat swab. In this test, antibody specific to Strep A carbohydrate antigen is coated on the test line region of the test. During testing, the extracted throat swab specimen reacts with an antibody to Strep A that is coated onto particles. The mixture migrates up the membrane to react with the antibody to Strep A on the membrane and generate a color line in the test line region. The presence of this color line in the test line region indicates a positive result, while its absence indicates a negative result. To serve as a procedural control, a colored line will always appear in the control line region, indicating that proper volume of specimen has been added and membrane wicking has occurred.
Product Detail
-
-
Brand:QL
Specimens: : Swab
Reading time:5 minutes.
Pack:20 T
STORAGE: 2‐30°C
KIT COMPONENTS(Device)
- •Test devices •Sterile swabs
- •Workstation • Strep A Reagent A (1M Sodium Nitrite )
- •Strep A Reagent B (0.4M Acetic Acid) • Test tubes
- •Dropper tips • Package insert
KIT COMPONENTS(strip)
- •Test strips • Sterile swabs
- •Workstation • Strep A Reagent A (1M Sodium Nitrite )
- •Strep A Reagent B (0.4M Acetic Acid) • Test tubes
- •Package insert
-
PROCEDURE
- Allow the test device, reagents, throat swab specimen, and/or controls to reach room temperature (15-30°C) prior to testing.
1. Remove the test device from the sealed foil pouch and use it as soon as possible.
Best results will be obtained if the test is performed immediately after opening the foil pouch.
2. Hold the Reagent A bottle vertically and add 4 full drops (approximately 240 µL) of Reagent A to an extraction test tube. Reagent A is red in color. Hold the
Reagent B bottle vertically and add 4 full drops (approximately 160 µL) to the tube. Reagent B is colorless. Mix the solution by gently swirling the extraction test tube. The addition of Reagent B to Reagent A changes the color of the solution from red to yellow.
3. Immediately add the throat swab to the extraction test tube of yellow solution. Agitate the swab 10 times in the tube. Leave the swab in the tube for 1 minute. Then press the swab against the side of the tube and squeeze the bottom of the tube as the swab is withdrawn. Discard the swab.
4. Fit the dropper tip on top of the extraction test tube. Place the test device on a clean and level surface. Add 3 full drops of solution (approx. 100 µL) to the specimen well (S) and then start the timer.
5. Wait for the colored line(s) to appear. Read the result at 5 minutes. Do not read the result after 10 minutes.
INTERPRETATION OF RESULTS
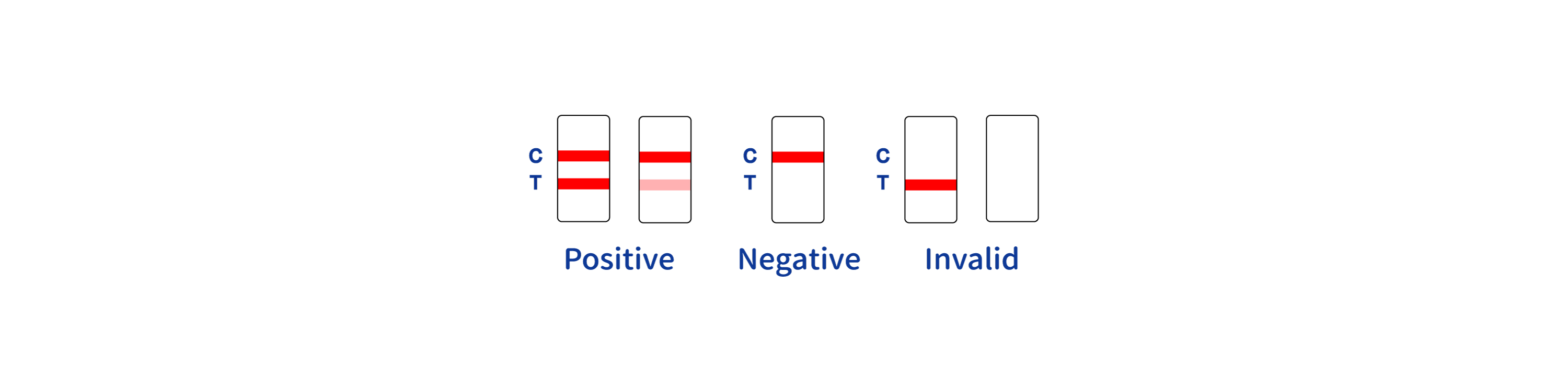
- NOTE:
- The intensity of the color in test region (T) may vary depending on the concentration of aimed substances present in the specimen. Therefore, any shade of color in the test region should be considered positive. Besides, the substances level can not be determined by this qualitative test.
- Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
Understanding the principle behind this groundbreaking product is crucial for healthcare professionals seeking to improve diagnostic workflows. The Rota/Adeno Combo Test operates on a lateral flow immunoassay mechanism, employing a unique blend of antibodies that are sensitive and specific to the Rota and Adeno viruses. When a stool sample is applied, it migrates through the device strip, encountering these antibodies. The presence of Rota or Adeno viral antigens triggers a visual signal, thus indicating a positive result with high accuracy. This not only facilitates a rapid response but also ensures that appropriate treatment interventions can be deployed promptly, minimizing the risk of disease spread and severity. The versatility and ease of use of the Rota/Adeno Combo Test make it an indispensable tool in various healthcare settings, from hospitals and clinics to remote field operations. Its design is oriented towards providing clear, straightforward results within minutes, eliminating the need for extensive laboratory equipment or specialized training. Whether it's for routine screenings or acute outbreak management, the Rota/Adeno Combo Test by QL Biotech is a reliable partner in the quest for enhanced diagnostic efficiency and improved patient care standards. QL Biotech remains committed to advancing healthcare diagnostics, one rapid test at a time, ensuring that communities worldwide benefit from prompt and accurate disease detection.


