SARS Coronavirus Test Device Manufacturer - QL Biotech
Product Main Parameters
| Parameter | Details |
|---|---|
| Brand | QL |
| Certificate | CE / TGA / ISO13485 / UK Registration |
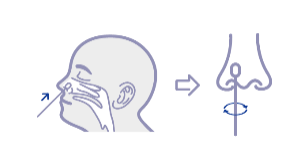
| Specimen | Nasal swab |
| Pack | 1 test, 5 tests, 20 tests |
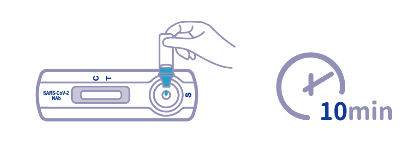
| Reading Time | 10 Minutes |
| Storage | 2-30℃ |
| Shelf Life | 2 Years |
Common Product Specifications
| Specification | Details |
|---|---|
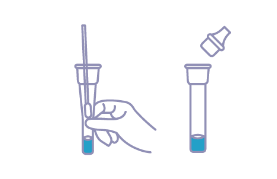
| Contents | Cassette, Sterile Nasal Swab, Buffer, Package Insert |
| Sensitivity | 90.91% |
| Specificity | 100.00% |
| Total Coincidence Rate | 98.21% |
Product Manufacturing Process
The manufacturing process of SARS Coronavirus Test Devices involves several critical stages to ensure high sensitivity and specificity. According to authoritative papers, the process begins with the formulation of specific reagents that can accurately bind to SARS-CoV-2 antigens. The reagents are then tested for their sensitivity to detect even minimal amounts of the virus. The device assembly process includes quality control measures where each batch of test devices is assessed for performance consistency. Advanced robotics and automated systems are often employed to minimize human error and contamination. The final products are then packaged under sterile conditions and stored based on validated stability protocols, ensuring a shelf life of up to two years. Overall, the careful attention to detail in the manufacturing process ensures that each SARS Coronavirus Test Device meets the high standards required for reliable diagnostics.
Product Application Scenarios
The application of SARS Coronavirus Test Devices is essential in various healthcare settings, particularly during pandemic responses. As documented in reputable studies, these devices are crucial for the timely diagnosis of COVID-19 in primary healthcare facilities, emergency departments, and community testing centers. The rapid and accurate detection of SARS-CoV-2 antigens allows healthcare professionals to manage patient isolation and treatment more effectively. Additionally, in occupational health settings, such as workplaces and schools, these devices help in monitoring health and preventing outbreaks. The ability to conduct self-tests at home offers an added layer of convenience for individuals, enabling them to make informed decisions about seeking medical care or isolating to prevent spread. Overall, the diverse application scenarios of SARS Coronavirus Test Devices highlight their significance in managing public health effectively.
Product After-Sales Service
- 24/7 customer support for product inquiries and troubleshooting advice.
- One-year warranty for product defects, excluding misuse or damage caused by improper handling.
- Replacement and refund services available for defective units within the warranty period.
- Comprehensive user manuals and video tutorials for product use and safety.
- Periodic updates and maintenance tips provided to optimize device performance.
Product Transportation
- Secure packaging to ensure product integrity during transportation.
- Partnerships with reliable logistics firms for timely and efficient delivery.
- Temperature-controlled shipping options to maintain optimal product storage conditions.
- Tracking services available for real-time monitoring of shipments.
- Environmentally friendly packaging materials used to minimize ecological footprint.
Product Advantages
- Rapid test results within 10 minutes.
- High specificity and sensitivity for accurate detection.
- Easy-to-use design suitable for self-testing.
- Cost-effective solution for mass screenings.
- Comprehensive support and after-sales service.
Product FAQ
- What is the primary use of the SARS Coronavirus Test Device?
The primary use of the SARS Coronavirus Test Device is to detect the presence of SARS-CoV-2 antigens from nasal swab specimens, enabling rapid and accurate diagnosis of COVID-19. - Who can use this test device?
The device is designed for individuals aged 15 to 70 years. Those unable to self-administer the test can be assisted by another adult. - How long does it take to get the results?
Results can be read within 10 minutes. It is crucial to interpret them within 20 minutes for accuracy. - What certifications does this product hold?
Our test device is certified by CE, TGA, ISO13485, and holds UK Registration, ensuring compliance with international standards. - How should the test device be stored?
The test device should be stored at temperatures between 2-30℃, maintaining its quality and effectiveness until the two-year shelf life. - What should I do if my test result is positive?
If the test result is positive, it is recommended to seek medical advice and follow local health guidelines, including isolation and further testing. - Can the test be reused?
No, the test device is designed for single use only to maintain hygiene and accuracy. - Is the device suitable for detecting other coronaviruses?
The device specifically detects antigens from SARS-CoV-2 and not other coronaviruses like MERS or the common cold. - How should I dispose of the test components?
Used components should be disposed of in a biohazard waste bag, following local regulations for hazardous waste disposal. - Can the test device be used in low-resource settings?
The design of the test device allows it to be used across various settings, including low-resource areas, due to its minimal equipment requirement and ease of use.
Product Hot Topics
- Impact of Rapid Testing on COVID-19 Management
Rapid testing for COVID-19 using devices such as the SARS Coronavirus Test Device has significantly impacted the management of the pandemic. Quick test results facilitate timely intervention measures, helping to isolate affected individuals promptly and reduce the potential for virus spread. The ability to conduct tests outside of traditional laboratory settings enhances accessibility, particularly in remote or underserved areas. The increased availability of rapid test devices has also supported public health efforts by enabling widespread screenings and data collection for tracking the virus's spread and mutations. - Challenges in SARS-CoV-2 Antigen Testing
While antigen tests, such as those provided by QL Biotech, offer rapid detection capabilities, challenges remain in ensuring consistent sensitivity and specificity across diverse populations. Variability in specimen collection techniques and the need for adequate viral load to trigger a positive result are critical factors affecting test accuracy. Quality control in manufacturing and adherence to proper testing procedures are thus essential to minimize false negatives or positives. Continued research and technological advancements are necessary to refine antigen test devices and enhance their reliability, especially in asymptomatic or low viral load cases. - Technological Advancements in SARS Coronavirus Test Devices
The development of SARS Coronavirus Test Devices has evolved significantly with technological advancements, enabling higher accuracy and faster results. Improved reagent formulations, automated manufacturing processes, and innovative detection methodologies have contributed to more reliable testing devices. Moreover, integration with digital platforms for results reporting and data analysis offers insights into virus trends and patterns. These advancements not only improve the efficiency of COVID-19 testing but also lay the groundwork for responses to future infectious disease outbreaks. - Role of Manufacturers in Quality Assurance
Manufacturers, such as QL Biotech, play a pivotal role in ensuring the quality and reliability of SARS Coronavirus Test Devices. Rigorous quality control protocols during production, coupled with continuous monitoring of device performance, are critical for maintaining test accuracy. Regulatory compliance with international standards, such as CE and ISO certifications, further affirms the manufacturer's commitment to quality. Such diligence is fundamental to building trust among consumers and healthcare providers, especially during public health emergencies where rapid and accurate diagnostics are paramount. - Global Accessibility of COVID-19 Test Devices
The global accessibility of COVID-19 test devices, including those manufactured by QL Biotech, remains a central challenge in pandemic management. Efforts to increase the distribution of test kits to low-resource regions are crucial for equitable healthcare provision. International collaboration and partnerships with local governments or NGOs may facilitate broader access, while cost-effective manufacturing and distribution strategies can make test devices more affordable. Bridging the accessibility gap ensures that all regions can benefit from rapid diagnostics and thus better control the virus spread. - The Economics of Mass Testing
The economics of mass testing, utilizing devices like the SARS Coronavirus Test Device, play a significant role in public health strategies. While initial setup and distribution may require substantial investment, the long-term benefits of curbing virus transmission and preventing healthcare system overload are considerable. Manufacturers contribute to cost management by optimizing production processes and material usage. The affordability of test devices subsequently enables broader adoption in community settings, supporting large-scale testing initiatives, and reinforcing public health defenses against COVID-19. - Understanding Test Device Sensitivity and Specificity
Sensitivity and specificity are crucial metrics for evaluating the performance of SARS Coronavirus Test Devices. Sensitivity refers to the test's ability to accurately identify individuals with the virus, minimizing false negatives. Specificity measures the capacity to correctly identify those without the virus, thereby reducing false positives. For manufacturers like QL Biotech, achieving a balanced high sensitivity and specificity ensures reliable diagnostic results, fostering trust in the testing process. These metrics also guide healthcare professionals in interpreting test results and making informed clinical decisions. - Future Prospects for SARS-CoV-2 Testing Technology
The future prospects for SARS-CoV-2 testing technology are promising, with ongoing research paving the way for innovative diagnostic solutions. Emerging technologies, such as CRISPR-based assays and next-generation sequencing, could offer even greater accuracy and speed. Integrating these advancements with existing platforms may enhance point-of-care testing capabilities, potentially reducing dependency on centralized laboratories. Manufacturers like QL Biotech are expected to play a crucial role in translating these scientific breakthroughs into market-ready products, further strengthening public health infrastructure. - Consumer Trust in Self-Testing Devices
Consumer trust in self-testing devices, like SARS Coronavirus Test Device from QL Biotech, is vital for successful pandemic control. Clear instructions, reliable performance, and prompt customer service contribute to building confidence among users. Transparent communication about the limitations and proper usage of the test devices helps manage expectations and ensures correct interpretation of results. As self-testing becomes more prevalent, fostering consumer trust involves not just product quality, but also effective education and support systems from manufacturers. - Adaptive Strategies in Test Device Manufacturing
Adaptive strategies in test device manufacturing have been key to addressing the evolving demands of the COVID-19 pandemic. Manufacturers have had to scale production rapidly while maintaining quality standards, often necessitating innovations in supply chain management and production processes. Flexibility in manufacturing allows for quick adaptation to new regulatory requirements or changes in testing protocols. For companies like QL Biotech, such strategies ensure that they remain responsive to global health needs, providing reliable test devices when and where they are most needed.
Image Description