Supplier of Adeno Rapid Diagnostic Test by QL Biotech
Product Main Parameters
| Parameter | Details |
|---|---|
| Method | Lateral flow immunoassay |
| Sample Types | Nasopharyngeal, throat, conjunctival swabs, stool |
| Reading Time | 10-15 minutes |
| Storage | 2-30°C |
Common Product Specifications
| Specification | Details |
|---|---|
| Pack Size | 25 tests per kit |
| Shelf Life | 12 months |
| Control Line | Included for validity |
Product Manufacturing Process
The manufacturing of the Adeno Rapid Diagnostic Test involves several critical steps to ensure reliability and accuracy. Initially, recombinant antibodies specific to adenovirus antigens are prepared and conjugated with colloidal gold. The test strips are then assembled, layering nitrocellulose with capture antibodies and assembling the conjugate pads. Precision is key, as the test line and control line must be distinct and easily interpretable. Rigorous quality control procedures are implemented, verifying the sensitivity and specificity of each batch. According to authoritative papers, optimizing these parameters ensures a rapid diagnostic tool that balances ease of use with diagnostic fidelity, aiding timely medical decisions.
Product Application Scenarios
Adeno Rapid Diagnostic Test is widely applicable in clinical settings, such as hospitals and outpatient clinics, especially where rapid diagnostic results are paramount. As per authoritative studies, the test is crucial in pediatric care where adenovirus infections are prevalent, facilitating quick identification and management. Additionally, in immunocompromised patients, swift adenovirus detection aids in tailoring specific antiviral therapies. The test's utility extends to monitoring outbreaks in high-density areas like schools or daycare facilities, providing frontline healthcare workers with a tool for immediate intervention and containment of viral spread.
Product After-Sales Service
- 24/7 Support via hotline and email
- Comprehensive user manual included
- Training sessions for healthcare providers
- Replacement and refund policy for defective kits
- Regular updates on best practices and new research findings
Product Transportation
- Shipped in temperature-controlled containers
- Compliant with international shipping standards
- Tracking options available for all orders
- Fast shipping options for urgent requirements
- Secure packaging to prevent damage during transit
Product Advantages
- High sensitivity and specificity for adenovirus detection
- Simple procedure with minimal training required
- Rapid results enabling quick medical decisions
- Non-invasive sample collection methods
- Applicable in a variety of healthcare settings
Product FAQ
- How accurate is the Adeno Rapid Diagnostic Test?
The Adeno Rapid Diagnostic Test, supplied by QL Biotech, is designed to deliver exceptionally accurate results with high sensitivity and specificity, minimizing false positives and negatives. However, it should be used alongside clinical evaluations for a more comprehensive diagnosis.
- What types of samples can be used?
The supplier Adeno Rapid Diagnostic Test supports various sample types including nasopharyngeal swabs, throat swabs, conjunctival swabs, and stool samples, providing flexibility based on symptoms and clinical presentation.
- How should the test be stored?
For optimal performance, store the Adeno Rapid Diagnostic Test at temperatures between 2-30°C. Ensure the storage area is dry and the tests remain in their sealed packaging until use.
- What is the shelf life of the test kits?
The Adeno Rapid Diagnostic Test kits from QL Biotech have a shelf life of 12 months from the manufacturing date, enabling stock flexibility for healthcare providers.
- Can this test be used in remote areas?
Yes, the Adeno Rapid Diagnostic Test is designed for use in various healthcare settings, including remote areas with limited access to comprehensive laboratory facilities, ensuring broad applicability and convenience for healthcare professionals.
- How are the results interpreted?
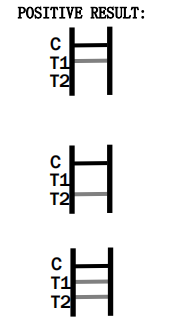
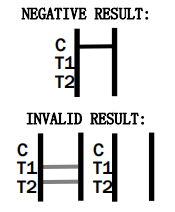
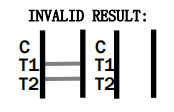
The test results are interpreted by checking the test and control lines on the strip. A visible line in the control region indicates the test has functioned correctly, while lines in the test regions indicate a positive result for adenovirus antigens.
- What precautions should be taken while using the test?
Ensure you read the instructions thoroughly before use, avoid using expired kits, and handle samples and test devices with care, following all biosafety guidelines to prevent contamination or erroneous results.
- Are there any limitations to the test?
While the Adeno Rapid Diagnostic Test offers rapid results, it may not detect all adenovirus serotypes. For ambiguous cases, further confirmatory testing through laboratory methods like PCR may be required to ensure diagnostic accuracy.
- What is the time frame for obtaining results?
Results from the supplier Adeno Rapid Diagnostic Test can be obtained within 10-15 minutes, facilitating immediate clinical decisions and patient management strategies.
- Is there customer support available for test-related inquiries?
Absolutely, QL Biotech provides comprehensive customer support for all Adeno Rapid Diagnostic Test-related inquiries, ensuring users receive the necessary assistance for troubleshooting and efficient test utilization.
Product Hot Topics
- Advancements in Rapid Diagnostic Testing
The field of rapid diagnostic testing continues to revolutionize healthcare, with suppliers like QL Biotech at the forefront. The Adeno Rapid Diagnostic Test epitomizes these advancements, combining speed and accuracy to empower healthcare providers with immediate diagnostic capabilities. As technology progresses, these tests will become even more integral to preventative healthcare and epidemic control.
- The Role of Rapid Testing in Pandemic Preparedness
Rapid diagnostic tests, such as the Adeno Rapid Diagnostic Test, play a crucial role in pandemic preparedness. They enable healthcare systems to quickly identify and respond to outbreaks, potentially curbing transmission rates. With a reliable supplier like QL Biotech, healthcare providers can maintain readiness and resilience in the face of emerging infectious threats.
- Integrating Rapid Tests in Routine Clinical Practice
Incorporating rapid diagnostic tests like the Adeno Rapid Diagnostic Test into routine clinical practice can significantly enhance patient management. By providing quick results, these tests facilitate immediate treatment adjustments, improving patient outcomes and streamlining clinic workflows. As they become more commonplace, the reliance on such suppliers will only grow.
- The Economic Impact of Rapid Diagnostics
Cost-efficiency is a major consideration in healthcare, and rapid diagnostics offer a compelling economic advantage. Tests like the Adeno Rapid Diagnostic Test can reduce unnecessary hospitalizations and treatments by providing precise and timely diagnoses. Suppliers like QL Biotech play a pivotal role in making these tests accessible and affordable.
- Ethical Considerations in Rapid Testing
As rapid testing becomes more pervasive, ethical considerations around accuracy, consent, and data privacy become paramount. Suppliers like QL Biotech are tasked with ensuring their tests meet rigorous standards to protect patient interests and maintain public trust in diagnostic technologies.
- Training Healthcare Staff on Rapid Test Usage
Efficient use of rapid diagnostic tests, such as the Adeno Rapid Diagnostic Test, hinges on proper training of healthcare staff. Suppliers like QL Biotech offer valuable training resources to ensure healthcare workers can utilize these tests correctly, achieving optimal patient outcomes and maintaining high standards of clinical care.
- Technological Innovations in Adenovirus Detection
The continuous evolution in adenovirus detection technology enhances the efficacy of tests like the Adeno Rapid Diagnostic Test. Suppliers are constantly improving these products to increase sensitivity and specificity. Staying abreast of these innovations is crucial for healthcare providers aiming to offer the best diagnostic services.
- Patient Compliance and Rapid Testing
Non-invasive and quick, rapid diagnostic tests increase patient compliance significantly. The Adeno Rapid Diagnostic Test exemplifies this, allowing patients to undergo testing without the discomfort associated with more invasive procedures, thereby enhancing patient satisfaction and engagement in their own healthcare.
- Rapid Testing and Antibiotic Stewardship
Rapid tests play a vital role in antibiotic stewardship by enabling accurate diagnoses that reduce unnecessary antibiotic prescriptions. The Adeno Rapid Diagnostic Test aids in distinguishing viral from bacterial infections, helping to combat antibiotic resistance — a growing global health issue.
- Future Prospects for Rapid Diagnostic Tests
The future of rapid diagnostic testing is bright, with continuous advancements promising even quicker and more accurate results. Suppliers like QL Biotech will be key players in this evolution, providing healthcare systems worldwide with cutting-edge solutions to meet diverse diagnostic needs.
Image Description