Supplier of Coronavirus IgG/IgM Diagnostic Test Device
Product Main Parameters
| Brand | QL |
|---|---|
| Certificate | CE |
| Specimen | Whole Blood/Serum/Plasma |
| Pack | 25 T |
| Reading Time | 15 minutes |
| Storage | 2-30℃ |
| Shelf Life | 2 years |
Common Product Specifications
| Contents | Cassette, buffer, disposable pipettes, package insert |
|---|
Product Manufacturing Process
Research indicates that the production of IgG/IgM test kits involves an intricate process of antigen coating and membrane blocking to ensure specific interaction with human antibodies. The process is typically conducted in controlled environments to maintain product efficacy. High-quality antigen-coated particles are prepared and embedded into test membranes to allow for the capillary action necessary for rapid immunochromatographic testing. Rigorous quality checks at each stage help maintain accuracy levels, ensuring the reliability of each kit. In conclusion, maintaining stringent quality control during manufacturing is essential for the production of reliable and accurate diagnostic tests.
Product Application Scenarios
According to authoritative sources, the IgG/IgM Diagnostic Test is used in various scenarios, including emergency settings for rapid screening, clinics for routine check-ups, and research laboratories for monitoring immune responses. These tests are instrumental in epidemiological studies to assess population immunity levels. In healthcare settings, they are valuable tools for determining recent or past exposure to COVID-19, thus aiding in quarantine decisions and treatment adjustments. Continuous advancements in diagnostic technology expand the scope of these tests, enhancing their utility in diverse medical and research fields.
Product After-Sales Service
We provide comprehensive after-sales support, including guidance on product usage, troubleshooting assistance, and product recalls if necessary. Our team is committed to ensuring customer satisfaction by offering solutions for any challenges faced during product application.
Product Transportation
The test kits are transported under specified conditions to maintain their integrity. We ensure that all transportation complies with relevant regulations and standards, preserving the efficacy of the test kits until they reach the end user.
Product Advantages
- Quick results within 15 minutes.
- Usable in diverse settings without sophisticated equipment.
- Highly convenient for large-scale screenings.
Product FAQ
What is the primary use of this test?
The primary use is to detect IgG and IgM antibodies against COVID-19 in human blood, serum, or plasma, aiding in diagnosing recent or past infections.
How accurate is the test?
While rapid tests are convenient, they may have lower sensitivity and specificity compared to laboratory-based tests. It's essential to use them as part of a comprehensive diagnostic approach.
Who can perform this test?
The test is intended for professional use only, typically performed by healthcare professionals in clinical settings.
Can this test detect current COVID-19 infection?
This test detects antibodies, not the presence of the virus itself. For current infections, molecular tests like RT-PCR are recommended.
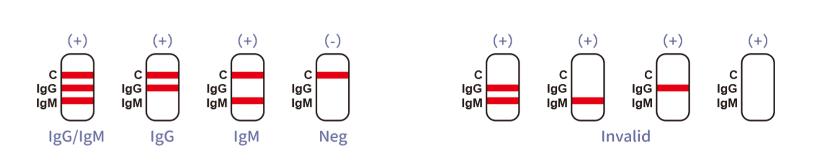
What does a positive IgM result indicate?
A positive IgM result suggests a recent infection, as IgM antibodies are the first to appear following exposure to the virus.
Is a positive IgG result indicative of immunity?
While IgG antibodies indicate a later stage of infection or past infection, immunity's duration and effectiveness remain a subject of ongoing research.
Can the test kits be reused?
No, the test kits are designed for single-use only to ensure accuracy and prevent cross-contamination.
How should the test kits be stored?
The test kits should be stored at a temperature range of 2-30°C to maintain their viability.
What precautions should be taken during the test?
Use personal protective equipment, and handle specimens as if they contain infectious agents. Follow standard procedures for specimen disposal.
What should be done if the control line does not appear?
If the control line fails to appear, the test is invalid, and it must be repeated with a new test device.
Product Hot Topics
Importance of Rapid Testing in Pandemic Control
Rapid testing plays a crucial role in managing pandemics by providing quick insights into the spread of infections. As a trusted supplier of Coronavirus IgG/IgM Diagnostic Tests, we contribute significantly to these efforts. Rapid test results are essential for timely decision-making and public health interventions, especially in settings where immediate isolation and treatment are possible. The ease of use and fast turnaround time helps manage patient flow in hospitals and clinics, reducing the potential spread of diseases. Our commitment as a supplier is to ensure that these vital tools reach healthcare providers promptly and efficiently, aiding in the global fight against COVID-19.
Challenges and Opportunities in Antibody Testing
Antibody testing presents both challenges and opportunities in understanding COVID-19 transmission. A reliable supplier, like us, offers high-quality Coronavirus IgG/IgM Diagnostic Tests that aid in population-level assessments of virus exposure and recovery. However, challenges such as varying sensitivity and specificity across different tests present hurdles. Leveraging advancements in diagnostic technology helps improve the reliability of results. As a supplier, we continually innovate to address these challenges, ensuring that our products meet rigorous standards, thereby enhancing the scope and reliability of antibody testing within diverse settings.
Interpreting IgG and IgM Results Post-Vaccination
Post-vaccination, the interpretation of IgG and IgM antibody tests requires careful consideration. As a trusted supplier, our Coronavirus IgG/IgM Diagnostic Tests are designed to detect natural infection responses, which can be differentiated from vaccine-induced antibodies through detailed analysis. Understanding the results aids in assessing vaccine efficacy and determining individuals’ immune responses. Our role as a supplier is to facilitate the availability of accurate testing tools to researchers and clinicians, enabling better insights into immunity and guiding vaccination strategies effectively.
Image Description