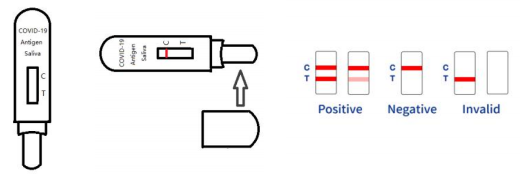
Supplier QL Biotech: Influenza A/B Rapid Test Device
Product Main Parameters
| Parameter | Details |
|---|---|
| Test Type | Immunoassay |
| Specimen Type | Nasal Swab |
| Detection Time | 10 minutes |
| Sensitivity | 90.91% |
| Specificity | 100% |
Common Product Specifications
| Specification | Details |
|---|---|
| Kit Size | 20 Tests/Kit |
| Storage | 15-30°C |
| Shelf Life | 12 Months |
Product Manufacturing Process
According to authoritative research, the manufacturing process of the Influenza A/B rapid test involves advanced techniques to ensure accuracy and reliability. The assay is built using a double antibody sandwich method, where monoclonal antibodies specific to Influenza A and B antigens are employed. These antibodies are coated onto a nitrocellulose membrane, allowing for the precise capture and detection of viral antigens from swab specimens. The process also involves rigorous quality control tests to maintain high-performance standards. This meticulous approach ensures that healthcare providers can confidently manage flu outbreaks with effective diagnostic tools.
Product Application Scenarios
Influenza A/B rapid test devices are crucial in various scenarios, as outlined in leading studies. They are widely used in clinical settings for the rapid identification of Influenza strains, aiding in timely patient management and outbreak control. In public health initiatives, these tests help in monitoring and curbing the spread of flu viruses within communities. Furthermore, the devices are essential during peak flu seasons, providing individuals with reliable testing options at point-of-care facilities. As a trusted supplier, QL Biotech ensures that these devices meet the highest standards of performance and reliability in all application scenarios.
Product After-Sales Service
QL Biotech offers comprehensive after-sales support, including user training, technical assistance, and product updates to ensure optimal performance of our Influenza A/B rapid test devices.
Product Transportation
Our products are carefully packaged to maintain integrity during transit, with reliable shipping partners ensuring timely delivery to suppliers globally.
Product Advantages
- Quick and reliable results in 10 minutes.
- High sensitivity and specificity for precise diagnosis.
- Easy-to-use format suitable for point-of-care testing.
- Comprehensive after-sales support and guidance.
Product FAQ
- How soon can results be obtained from the test?
The Influenza A/B rapid test provides results in approximately 10 minutes, making it ideal for quick diagnosis and timely treatment decisions.
- What type of specimen is required for testing?
The test requires a nasal swab specimen, which is easy to collect and minimally invasive for the patient.
- What is the shelf life of the test kit?
Each test kit has a shelf life of 12 months, provided it is stored under the recommended conditions between 15-30°C.
- Is the test suitable for use in children?
Yes, the Influenza A/B rapid test is suitable for use in children, with proper handling and collection of nasal swab specimens as indicated.
- How should the test be stored?
The test should be stored in a dry environment at a temperature range of 15-30°C to maintain its effectiveness throughout its shelf life.
- What are the advantages of using this test?
The test offers quick and accurate detection of Influenza A/B, aiding in the management and control of flu outbreaks with ease.
- Can the test detect subtypes of Influenza A?
The test is designed to detect Influenza A and B viruses, with high sensitivity, helping to identify the presence of these viruses in patients.
- Who can use the test kits provided by QL Biotech?
Our test kits are intended for use by healthcare professionals as well as point-of-care facilities, ensuring accessibility and user-friendliness.
- What support is available after purchasing the test?
QL Biotech offers comprehensive support including technical assistance, user guides, and updates to ensure optimal use of the product.
- Are there any interfering substances that may affect test results?
While the test is highly specific, certain substances may interfere if present in high concentrations. Proper specimen collection and handling are crucial.
Product Hot Topics
- How does the Influenza A/B rapid test contribute to public health?
The Influenza A/B rapid test is a critical tool in public health efforts to monitor and control flu outbreaks. By providing quick and accurate results, it allows healthcare providers to identify and isolate cases promptly, reducing the spread of the virus. The test is especially valuable during flu season when timely intervention is crucial to protect vulnerable populations. As a leading supplier, QL Biotech ensures that our tests meet the highest standards, supporting public health initiatives worldwide.
- Challenges in Influenza Diagnosis and the Role of Rapid Testing
Diagnosing Influenza can be challenging due to overlapping symptoms with other respiratory illnesses. Rapid testing plays a vital role in providing clarity and aiding healthcare providers in making informed treatment decisions. The specificity and sensitivity of tests like those provided by QL Biotech ensure that patients receive appropriate care promptly. As a result, rapid testing contributes to better patient outcomes and efficient management of healthcare resources.
- The Importance of Accurate Influenza Testing
Accurate Influenza testing is essential for managing flu outbreaks and minimizing their impact on communities. Rapid tests such as those supplied by QL Biotech enable timely diagnosis, which is critical for effective treatment and reducing transmission. By ensuring high accuracy, these tests help healthcare providers distinguish between different viral infections, ultimately improving patient care and public health responses.
- Advancements in Rapid Testing for Influenza A/B
Recent advancements have led to improved accuracy and user-friendliness of rapid tests for Influenza A/B detection. Innovations in immunoassay technology have enhanced the performance of these tests, making them indispensable tools in clinical settings. As a trusted supplier, QL Biotech is at the forefront of these developments, offering products that meet the evolving needs of healthcare providers and patients.
- Addressing Seasonal Flu Surges with Rapid Testing
During seasonal flu surges, rapid testing becomes a frontline tool for healthcare providers. The efficiency and reliability of tests supplied by QL Biotech allow for quick identification and management of cases, preventing further spread. Our products are designed to support healthcare systems by providing accurate results, thus enhancing patient care and public health preparedness during peak flu seasons.
- Ensuring Supply Chain Stability for Diagnostic Kits
QL Biotech prioritizes supply chain stability to ensure uninterrupted availability of our diagnostic kits. By maintaining robust distribution networks and proactive planning, we cater to the demands of healthcare facilities globally. Our commitment as a leading supplier is to deliver timely solutions that meet the diagnostic needs during critical influenza seasons.
- Impact of Rapid Testing on Patient Management
Rapid testing significantly influences patient management by enabling quick diagnosis and treatment decisions. With reliable tests like those from QL Biotech, healthcare providers can efficiently differentiate Influenza A/B infections, ensuring timely intervention. This approach not only improves individual patient outcomes but also aids in controlling community-level spread of the virus.
- How Influenza A/B Tests Assist Disease Surveillance
Influenza A/B testing aids in disease surveillance by providing accurate data on virus prevalence within communities. This information is crucial for public health officials in tracking flu trends and planning appropriate response strategies. As a premier supplier, QL Biotech supports these efforts with tests that deliver reliable results, contributing to effective flu management strategies globally.
- Preparing Healthcare Facilities for Influenza Season
Healthcare facilities must be prepared for influenza season, and having reliable testing equipment is key. Rapid tests supplied by QL Biotech are designed to support such preparations, offering high accuracy and convenience. By equipping facilities with efficient diagnostic tools, we help ensure readiness for potential influenza outbreaks, thus safeguarding public health.
- Benefits of Partnerships with Suppliers for Flu Management
Partnering with reputable suppliers like QL Biotech enhances flu management capabilities for healthcare facilities. Such partnerships ensure access to reliable, high-quality testing kits essential for accurate diagnosis and timely intervention. By leveraging supplier expertise, healthcare providers can improve their preparedness and response to influenza outbreaks, ultimately benefiting public health.
Image Description