Swift and Accurate Influenza A+B/COVID/RSV COMBO RAPID TEST by QL Biotech
PRODUCT SPECIFICATION
|
Brand |
QL |
Certificate |
CE / TGA / ISO13485/UK Registration |
|
Specimen |
Nasal swab |
Pack |
1 test 5 tests 20 tests |
|
Reading Time |
10 Minutes |
Contents |
Cassette, Sterile Nasal Swab, Buffer ,Package Insert |
|
Storage |
2-30℃ |
Shelf Life |
2 Years |
Specimen Collection
It is important to obtain as much secretion as possible. Therefore, to collect a nasal swab sample, insert the sterile swab into the nostril that presents the most secretion under visual inspection. Using gentle rotation, push the swab until resistance is met at the level of the turbinates (less than one inch into the nostril). rotate the swab 8-10s for five times at least against nasal wail.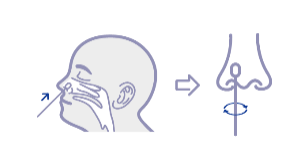
PROCEDURE
Bring tests, specimens, and/or controls to room temperature (15-30°C) before use.
- ● Wear the protection equipment. Remove the test from its sealed pouch, and place it on a clean, level surface. For best results, the assay should be performed within one hour.
Place the swab specimen into the Extraction Tube. Roll the swab at least 10 times while pressing the swab against the bottom and side of the Extraction Tube. Roll the swab head against the inside of the Extraction Tube as you remove it. Try to release as much liquid as possible. Dispose of the used swab into the Disposal Bag considered biohazard waste.
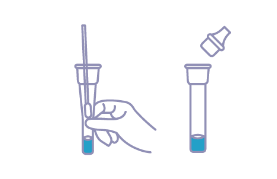
● Put on the tube tip, then add 3 drops (100ul) of extracted sample into the sample well (S). Do not handle or move the Test Device until the test is complete and ready for reading.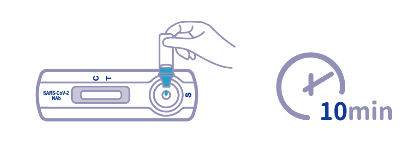
- ● As the test begins to work, color will migrate across the membrane. Wait for the colored band(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 20 minutes.
- ● Dispose all assayed equipment (cassette, buffer and so on) into the Disposal Bagconsidered as biohazard.
PERFORMANCE CHARACTERISTICS
Test results of the candidate kit and the reference kit are summarized in 2×2 table below.
|
|
Vitassay qPCR SARS-CoV-2 |
Total |
||
|
Positive |
Negative |
|||
|
SARS-CoV-2 Antigen Rapid Test Device |
Positive |
100 |
0 |
100 |
|
Negative |
10 |
450 |
460 |
|
|
Total |
110 |
450 |
560 |
|
|
Statistic |
Value |
95% CI |
|
Sensitivity |
90.91% |
(83.92%~95.55%) |
|
Specificity |
100.00% |
(99.18%~100.00%) |
|
Total coincidence rate |
98.21% |
(96.74%~99.14%) |
The 95% confidence intervals of sensitivity, specificity, the total coincidence rate is calculated based on the binomial distribution.
It is important to note that specimen collection is a vital part of the process, and it is crucial to collect as much nasal secretion as possible to ensure accurate results. Clear instructions are provided with the kit, guiding users through the collection process to maximize accuracy. Overall, our Influenza A+B/COVID/RSV COMBO RAPID TEST stands out as an embodiment of QL Biotech's commitment to providing cutting-edge, reliable, and convenient diagnostic solutions. Trust in QL Biotech for swift answers when you need them most.




