Wholesale HCV Rapid Test CE Marked for Antibody Detection
Product Main Parameters
| Parameter | Specification |
|---|---|
| Detection | HCV Antibodies |
| Sample Type | Whole Blood/Serum/Plasma |
| Result Time | 15 minutes |
| Storage | 2-30°C |
| Pack Size | 25 Tests |
Common Product Specifications
| Specification | Detail |
|---|---|
| CE Marked | Yes |
| Portability | Compact Design |
| Usage | In Vitro Diagnostic Use |
| Stability | Do not freeze |
Product Manufacturing Process
The manufacturing process of the HCV Rapid Test involves rigorous quality controls and advanced technologies to ensure consistency and accuracy. Each test is produced in a sterile environment, adhering to international standards. The test strips are assembled with precision, integrating membrane strips coated with specific antigens. The process involves the application of conjugates and the sealing of devices in protective pouches. Final products undergo extensive testing to confirm functionality, reliability, and compliance with the CE marking requirements, ensuring they meet the necessary safety and performance standards required for wholesale HCV Rapid Test CE Marked distribution.
Product Application Scenarios
The HCV Rapid Test CE Marked is designed for diverse application scenarios. It is ideal for use in clinical laboratories, hospitals, and remote healthcare settings where rapid diagnosis is essential. The test's ease of use and quick results make it suitable for emergency rooms, outpatient clinics, and mobile health units. It is particularly effective in resource-limited settings where access to comprehensive laboratory facilities is restricted. The portability and affordability of the test support large-scale screening programs, enhancing early diagnosis and treatment interventions in various populations.
Product After-Sales Service
We provide comprehensive after-sales support for our wholesale HCV Rapid Test CE Marked products, including technical assistance, training for healthcare professionals, and a warranty for defective items. Our dedicated customer service team is available to address any inquiries and ensure satisfaction with the product.
Product Transportation
The HCV Rapid Test kits are shipped in climate-controlled vehicles to maintain optimal temperature conditions. Packaging is designed to protect the test devices from physical damage and exposure to moisture, ensuring they arrive in perfect condition.
Product Advantages
- CE Marked Assurance: Ensures compliance with EU safety standards.
- Rapid Results: Provides results in just 15 minutes, facilitating swift clinical decisions.
- User-Friendly: Can be administered with minimal training, increasing accessibility.
- Cost-Effective: A financially viable option for mass screening.
- Portability: Compact design allows easy distribution and use in diverse locations.
Product FAQ
- What is the purpose of the HCV Rapid Test?
The HCV Rapid Test CE Marked is designed for the qualitative detection of Hepatitis C virus antibodies in human blood, serum, or plasma. It aids in the diagnosis of HCV infection by providing rapid results.
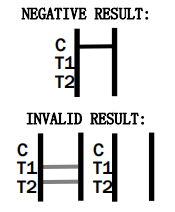
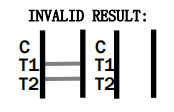
- How accurate is the HCV Rapid Test CE Marked?
The test is designed to be highly accurate, adhering to EU safety and performance standards. However, confirmatory testing is recommended following a positive result.
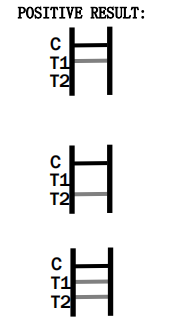
- What should I do if the test result is positive?
If the HCV Rapid Test shows a positive result, it is advisable to consult a healthcare professional for confirmatory testing and appropriate medical advice.
- Can the test be used in non-laboratory settings?
Yes, the test is portable and user-friendly, making it suitable for use in a variety of environments, including clinics, mobile health units, and remote areas.
- What is the shelf life of the test?
The test devices have a shelf life indicated on the packaging. It is important to store them as instructed and not to use them beyond their expiration date.
- Is the test safe for pregnant women?
The test is safe for use in all individuals, including pregnant women, as it is non-invasive and used for diagnostic purposes only.
- How should the test be stored?
Store the test kits in a dry place at temperatures between 2-30°C. Do not freeze the tests as this can affect their performance.
- Can the test detect early HCV infection?
The test detects antibodies, which may not appear in early infection stages. Confirmatory tests are recommended for early detection.
- What training is needed?
Minimal training is required to administer the test, as it is designed for ease of use with clear instructions provided in the package.
- What should be done with used tests?
Dispose of used test devices following local regulations for biomedical waste to ensure safety and environmental protection.
Product Hot Topics
- Is the HCV Rapid Test CE Marked efficient for mass screenings?
The HCV Rapid Test CE Marked is highly efficient for mass screenings due to its speed, accuracy, and cost-effectiveness. It is a preferred choice in public health initiatives targeting Hepatitis C control and prevention.
- How does the HCV Rapid Test compare to traditional lab tests?
While traditional lab tests are highly accurate, they often take longer to process. The HCV Rapid Test CE Marked provides results quickly, making it ideal for immediate decision-making, especially in critical care settings.
- What considerations are there for using the test in rural areas?
In rural areas, the test's portability, ease of use, and minimal training requirements make it an excellent choice, providing access to essential diagnostic capabilities where traditional lab services may be unavailable.
- How is the test CE Marked?
The CE Mark on the HCV Rapid Test indicates that it meets EU safety, health, and environmental protection requirements. This certification assures users of the test's reliability and quality.
- How environmentally friendly are the test kits?
The test kits are designed to minimize environmental impact, using materials that can be disposed of safely. Efforts are made to ensure packaging and waste management adhere to environmental standards.
- Can the test be used in emergency situations?
Yes, the rapid response capability of the test makes it suitable for emergency healthcare scenarios, providing quick results essential for timely medical intervention.
- What impact does the test have on healthcare cost management?
The affordability of the HCV Rapid Test CE Marked reduces overall healthcare costs related to Hepatitis C screening, making it a viable option for large-scale health programs and budget-conscious operations.
- What is the role of the HCV Rapid Test in global health?
The test plays a crucial role in global health by facilitating early detection of HCV, which is vital for controlling the spread of the virus and improving patient outcomes worldwide.
- Are there any restrictions on the use of the test?
The test is intended for in vitro diagnostic use only and should not be taken internally. Always follow the instructions provided for accurate results.
- What technological advancements are integrated into the test?
The HCV Rapid Test incorporates advanced lateral flow immunoassay technology, ensuring rapid and reliable detection of antibodies, further enhanced by its CE Mark certification assuring safety and efficacy.
Image Description