Wholesale Influenza AB/COVID/RSV Combo Rapid Test
Product Main Parameters
| Brand | QL Biotech |
|---|---|
| Certificate | CE / TGA / ISO13485 / UK Registration |
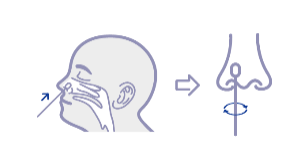
| Specimen | Nasal swab |
| Pack | 1 test, 5 tests, 20 tests |
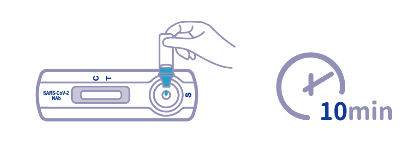
| Reading Time | 10 Minutes |
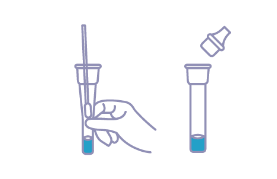
| Contents | Cassette, Sterile Nasal Swab, Buffer, Package Insert |
| Storage | 2-30℃ |
| Shelf Life | 2 Years |
Common Product Specifications
| Sensitivity | 90.91% (83.92%~95.55%) |
|---|---|
| Specificity | 100.00% (99.18%~100.00%) |
| Total Coincidence Rate | 98.21% (96.74%~99.14%) |
Product Manufacturing Process
The manufacturing of the Influenza AB/COVID/RSV Combo Rapid Test involves a precise process where lateral flow assay technology is employed. This involves embedding specific antibodies on a test strip that binds to viral antigens present in the sample. The process ensures high specificity and sensitivity by using high-quality reagents and robust quality control measures, ensuring consistency and reliability in each batch produced. This technology is validated by various studies demonstrating its efficiency in rapid viral detection, which is crucial for timely clinical decision-making during respiratory illness outbreaks.
Product Application Scenarios
The application of the Influenza AB/COVID/RSV Combo Rapid Test is particularly vital in clinical settings during peak respiratory illness seasons. It enables healthcare providers to swiftly identify the infection responsible for a patient's symptoms, enhancing patient management by informing the appropriate treatment strategies and public health responses. Furthermore, its application extends to emergency departments, outpatient clinics, and potentially at-home testing scenarios, provided adequate guidance is followed, thereby decentralizing testing capacities and improving accessibility to vital diagnostic tools during widespread outbreaks.
Product After-Sales Service
QL Biotech offers comprehensive after-sales services, ensuring customer satisfaction with the Influenza AB/COVID/RSV Combo Rapid Test. Customers can access technical support for product usage guidance and troubleshooting. Additionally, the company provides training resources to enhance users' proficiency with the test kits. In the case of defective products, QL Biotech ensures efficient replacement or refund procedures, prioritizing customer experience and maintaining product standards through continuous feedback and improvement processes.
Product Transportation
The Influenza AB/COVID/RSV Combo Rapid Test kits are transported under controlled environmental conditions to preserve their integrity and efficacy. Ensuring temperature stability (2-30℃) during transit is crucial, which is achieved through specialized packaging solutions that comply with industry regulations and standards for medical supplies. This meticulous approach guarantees that the kits arrive at their destination in optimal condition, ready for immediate use in clinical and diagnostic settings.
Product Advantages
- Speed and Convenience: Results in 15-30 minutes.
- Comprehensive Diagnosis: Simultaneous detection of multiple viruses.
- Improved Patient Management: Quick diagnosis aids in effective treatment planning.
Product FAQ
- What is the minimum age for self-testing with this kit?Individuals aged 15-70 can self-test using the kit. For those unable to do so, assistance from another adult is recommended.
- How does the test detect multiple viruses?The test uses immunoassay technology to detect virus-specific antigens through a color change on the test strip.
- Is the test suitable for at-home use?Yes, the test is designed for at-home use with detailed instructions included for proper sample collection and testing.
- What is the shelf life of the test kit?The test kit has a shelf life of 2 years when stored between 2-30°C.
- How should used test components be disposed of?Used components should be placed in the provided Disposal Bag and treated as biohazard waste.
- Can this test detect COVID-19 variants?Emerging variants could potentially affect detection if the antigens are not updated; ongoing research is required to maintain accuracy.
- Is the test CE certified?Yes, the test holds CE certification, along with other certifications like TGA and ISO13485.
- How accurate is the test?The sensitivity is 90.91% and specificity is 100%, ensuring high accuracy with a total coincidence rate of 98.21%.
- Can the test differentiate between influenza A and B?Yes, the test can differentiate between influenza A and B as well as COVID-19 and RSV.
- What are the temperature requirements for storing the test?The test should be stored in temperatures between 2-30℃ to maintain its efficacy.
Product Hot Topics
- Importance of Rapid Testing for Respiratory InfectionsIn the face of respiratory illnesses, rapid testing is pivotal. The Influenza AB/COVID/RSV Combo Rapid Test represents an advancement in diagnostic technology. By enabling quick on-the-spot diagnosis, healthcare providers can implement effective treatment strategies, improving outcomes and curbing transmission rates. These tests serve as an indispensable tool in managing seasonal outbreaks and pandemics, and their availability in wholesale enables broad distribution, improving access and response times in varied healthcare settings.
- Challenges in Differentiating Respiratory Virus SymptomsDifferentiating symptoms of influenza, COVID-19, and RSV is challenging due to their overlapping nature. The Influenza AB/COVID/RSV Combo Rapid Test offers a solution by identifying the specific virus responsible for symptoms within minutes. By offering this test wholesale, healthcare facilities can efficiently tackle diagnostics, reducing the need for multiple tests, conserving resources, and enabling faster clinical decisions.
Image Description