Wholesale New Coronavirus Quick Check Combo Rapid Test
Product Main Parameters
| Brand | QL |
|---|---|
| Certificate | CE |
| Specimen | Nasopharyngeal swabs/Nasal swab |
| Pack | 20T |
| Reading Time | 10 minutes |
| Contents | Cassette, Buffer, Package insert |
| Storage | 2-30℃ |
| Shelf Life | 2 years |
Common Product Specifications
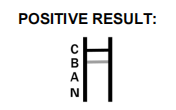
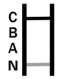
| Flu AB Positive | Colored bands in A, B, C regions |
|---|---|
| COVID-19 Positive | Colored bands in N, C regions |
| Negative Result | Only control band (C) |
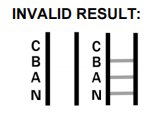
| Invalid Result | No control band (C) |
Product Manufacturing Process
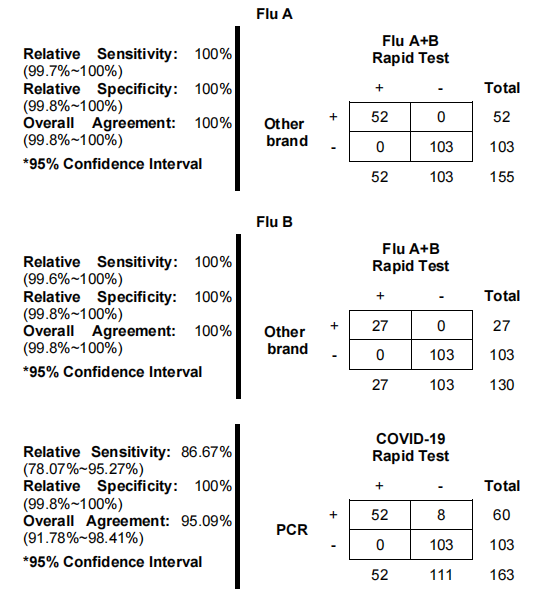
The manufacturing process of the New Coronavirus Quick Check involves rigorous quality control and precision engineering to ensure reliable and accurate detection of antigens. The process typically includes antibody production, conjugation, and assembly of test cassettes. According to authoritative studies, maintaining stringent conditions during antibody conjugation and incorporating high-affinity antibodies are crucial for test reliability. These procedures help achieve high specificity and sensitivity, essential for detecting viral antigens in clinical samples.
Product Application Scenarios
New Coronavirus Quick Check is especially beneficial for public health management, travel, and education sectors. Studies highlight its role in rapid decision-making, reducing time from sampling to diagnosis significantly compared to traditional methods. The test's ease of use and quick turnaround make it ideal for screening in schools, workplaces, and mass gathering events, facilitating immediate isolation measures and minimizing transmission risks.
Product After-Sales Service
QL Biotech offers comprehensive after-sales support, ensuring customer satisfaction and product efficacy. Services include troubleshooting assistance, replacement for defective units, and continual updates on product enhancements.
Product Transportation
The New Coronavirus Quick Check is shipped under controlled conditions to ensure product integrity. Packaging is designed to maintain temperature stability, preventing component degradation during transit.
Product Advantages
- Fast and reliable results within 10 minutes.
- Convenient packaging for ease of use in multiple settings.
- CE certified for trusted performance.
- Extended shelf life of 2 years under optimal storage conditions.
Product FAQ
- What is the detection method of this test?
The New Coronavirus Quick Check uses lateral flow immunoassay technology for detecting viral antigens in swab specimens. This provides a qualitative evaluation of infection status.
- How should samples be collected?
Samples should be collected using nasopharyngeal or nasal swabs following the guidelines provided in the manual to ensure accuracy and safety.
- Can the test detect asymptomatic infections?
While rapid antigen tests are effective, they may have lower sensitivity for asymptomatic infections compared to PCR tests. In such cases, confirmatory testing is recommended.
- What should be done in case of an invalid result?
An invalid test result indicates a failure in the testing process. It is advised to review the test procedure and repeat the test with a new kit. Contact support if issues persist.
- Is the test reusable?
No, the New Coronavirus Quick Check is designed for single use only. Proper disposal of used tests according to local regulations is important.
- What is the importance of the control band?
The control band validates the test process. Its appearance confirms the test has been performed correctly. No control band indicates an invalid test.
- Can the test be used for self-testing?
The test is intended for professional use. Self-testing should be done under guidance to ensure proper sample collection and result interpretation.
- What are the storage conditions for the test?
The test should be stored between 2-30℃. Avoid exposure to direct sunlight and moisture to maintain its efficacy over the shelf life.
- How is the test useful for public health management?
By providing quick results, it enables timely interventions to control the spread of infection, especially during outbreaks in communities.
- Is the test CE certified?
Yes, the New Coronavirus Quick Check is CE certified, ensuring compliance with safety and efficacy standards within the European Union.
Product Hot Topics
- Understanding the New Coronavirus Quick Check Technology
The New Coronavirus Quick Check utilizes advanced lateral flow technology to detect specific antigens related to COVID-19 and Influenza viruses. This ensures rapid identification, crucial for managing potential outbreaks efficiently.
- The Role of Rapid Testing in Pandemic Management
The introduction of rapid tests like the New Coronavirus Quick Check has been a game-changer in managing the COVID-19 pandemic. By providing quick results, these tests aid in reducing transmission rates through timely isolation of infected individuals.
- Comparing Rapid Antigen Tests and PCR Testing
While PCR tests are highly sensitive, rapid antigen tests like the New Coronavirus Quick Check offer the advantage of speed and convenience. These tests are particularly valuable in scenarios requiring frequent and immediate testing.
- Impact of Rapid Tests on Workplace Safety
Implementing regular testing with the New Coronavirus Quick Check in workplaces helps maintain safe environments, reducing the risk of outbreaks and ensuring business continuity.
- Choosing the Right Rapid Test for Your Needs
When selecting a rapid test, factors like sensitivity, specificity, and certification are key. The New Coronavirus Quick Check provides reliable performance, supported by CE certification.
- Exploring Future Trends in Diagnostic Testing
As diagnostic technology evolves, we expect even more accurate and faster testing options. The New Coronavirus Quick Check represents the current forefront of this evolution, balancing speed and reliability.
- The Science Behind Antigen Detection
Antigen detection tests identify specific proteins from pathogens. The New Coronavirus Quick Check targets these proteins, allowing for rapid diagnosis of infections, which is essential during a pandemic.
- Cost-Effective Solutions for Mass Testing
The affordability of the New Coronavirus Quick Check makes it a practical solution for mass testing in schools and communities, ensuring broad access to essential health services.
- Adapting Testing Strategies in Response to New Variants
As new virus variants emerge, rapid tests like the New Coronavirus Quick Check must adapt to these changes. Continuous updates to antibody targets are crucial for maintaining test efficacy.
- Enhancing Public Confidence in Rapid Tests
Building trust in rapid test results is essential for public health strategies. By ensuring high accuracy and reliability, tests like the New Coronavirus Quick Check play a vital role in pandemic management.
Image Description