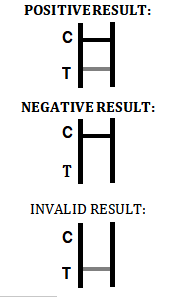China iGFBP-1 Rapid Test Device for Vaginal Secretion
Product Main Parameters
| Product Name | iGFBP-1 Rapid Test Device |
|---|---|
| Usage | Professional in vitro diagnostic use only |
| Specimen | Vaginal secretion |
| Packing | 20 tests/box, 1 test/polybag |
| MOQ | 1000 tests |
Common Product Specifications
| Storage Temperature | 2‐30°C |
|---|---|
| Stability | Until the expiry date on the pouch |
| Freezing | Do not freeze |
Product Manufacturing Process
The manufacturing of the iGFBP-1 Rapid Test Device involves a precise immunochromatographic technique. This method is designed to ensure specificity and sensitivity in detecting the iGFBP-1 marker in vaginal secretions. The process involves the careful conjugation of antibodies to gold particles, ensuring that the test can accurately detect the presence of the iGFBP-1 protein. The final product is rigorously tested for quality assurance, adhering to international standards. This ensures that the test provides reliable results for diagnosing the rupture of fetal membranes, a critical assessment in pregnancy.
Product Application Scenarios
The iGFBP-1 Rapid Test Device is primarily used in clinical settings to diagnose the rupture of fetal membranes (ROM) in pregnant women. This condition, if left undiagnosed, can lead to complications such as infections or preterm birth. By providing a quick and reliable diagnostic tool, healthcare providers in China can make informed decisions regarding the management of pregnancies. The test is easy to use and provides results within a short timeframe, allowing for timely interventions. Its application in prenatal care significantly enhances maternal and fetal health outcomes, contributing to a safer pregnancy journey.
Product After-sales Service
Our after-sales service includes comprehensive customer support, ensuring users have access to technical assistance and detailed documentation for the iGFBP-1 Rapid Test Device. We offer troubleshooting guidance and replacement for defective products.
Product Transportation
Products are carefully packed to ensure stability during transportation. They are shipped via reliable carriers, maintaining optimal conditions (2‐30°C) from our facilities in China to the destination.
Product Advantages
- High specificity for iGFBP-1 detection.
- Quick and reliable results.
- Simple and easy to use.
- Cost-effective solution for ROM diagnosis.
- Comprehensive after-sales support.
Product FAQ
- What is the iGFBP-1 Rapid Test Device used for?
The iGFBP-1 Rapid Test Device is used for detecting the presence of insulin-like growth factor-binding protein 1 (iGFBP-1) in vaginal secretions. This helps diagnose the rupture of fetal membranes (ROM) in pregnant women, a condition that requires immediate medical attention to prevent complications such as infections or preterm labor. - How does the iGFBP-1 test work?
This test utilizes immunochromatography to detect the presence of iGFBP-1 in vaginal secretions. It involves a dipstick that shows a color change when the protein is detected, indicating a positive result for ROM. The control band ensures the test has been conducted correctly, providing reliable results. - Can the test be used at home?
No, the iGFBP-1 Rapid Test Device is designed for professional in vitro diagnostic use only. It should be administered by healthcare professionals in a clinical setting to ensure accurate interpretation and appropriate follow-up care. - What are the storage conditions for the test?
The test should be stored at a temperature range of 2‐30°C and must remain in its sealed pouch until use. Freezing the test is not recommended as it may affect the test's accuracy and reliability. - What should I do if I get an invalid result?
An invalid result occurs when the control band fails to appear. In this case, review the procedure and repeat the test with a new device. If the issue persists, discontinue using the kit and contact customer support for assistance. - How can I order the iGFBP-1 Rapid Test Device?
The minimum order quantity (MOQ) is 1000 tests. Orders can be placed through our official website or by contacting our sales team directly. We offer various cooperation modes, including OEM/ODM options, to meet the specific needs of our clients. - Are there any known factors that can affect the test results?
Yes, factors such as insufficient specimen volume, incorrect test procedure, or expired tests can lead to inaccurate results. It's crucial to follow the instructions carefully to ensure the reliability of the test outcome. - Is the test CE marked or FDA approved?
The regulatory status of the test can be confirmed on the product's packaging or by contacting our sales team. We strive to meet and maintain compliance with relevant international standards and certifications. - How long does it take to get test results?
The iGFBP-1 Rapid Test Device provides results within minutes, allowing healthcare providers to make timely decisions. The rapid response time is crucial for managing pregnancies where ROM is suspected. - What are the potential risks of not diagnosing ROM?
Failure to diagnose the rupture of fetal membranes can lead to serious complications, including infections and preterm birth. Prompt diagnosis through the iGFBP-1 test enables appropriate medical interventions, reducing risks to both the mother and fetus.
Product Hot Topics
- Advancements in iGFBP-1 Testing in China
The development of rapid diagnostic tests like the iGFBP-1 device represents a significant advancement in prenatal healthcare in China. Such innovations improve clinical outcomes by providing healthcare professionals with quick and accurate tools for diagnosing conditions like the rupture of fetal membranes (ROM). The ease of use and reliability of these tests underscore the progress in medical technology aimed at enhancing patient care. With a focus on patient safety, these advancements are setting new standards in prenatal diagnostics. - China's Impact on Global Diagnostic Innovations
China's role as a leader in medical diagnostics continues to grow, with companies like Zhejiang QL Biotech Co., Ltd contributing significantly to global innovations. The iGFBP-1 Rapid Test Device exemplifies the quality and precision that Chinese manufacturers bring to the table. As demand for reliable diagnostic tools increases worldwide, China's contributions remain pivotal in ensuring accessibility and affordability, particularly in areas where rapid and accurate diagnosis can save lives. - Understanding iGFBP and Its Clinical Significance
Insulin-like growth factor-binding proteins (iGFBP) play a crucial role in modulating the activity of IGFs, impacting growth and development. In clinical diagnostics, tests like the iGFBP-1 Rapid Test Device are essential for assessing conditions like ROM in pregnant women. Understanding the function of iGFBP enhances the ability to diagnose and manage various health conditions, emphasizing the importance of continued research and innovation in this field. China's focus on such advancements highlights the commitment to improving healthcare outcomes globally. - The Future of Prenatal Diagnostics in China
The landscape of prenatal diagnostics is rapidly evolving, with China at the forefront of developing innovative solutions. The iGFBP-1 Rapid Test Device represents the future of prenatal care, providing accurate and rapid results to detect critical conditions like the rupture of fetal membranes. As technology advances, the emphasis on non-invasive, efficient, and reliable diagnostic tools continues to grow, paving the way for improved maternal and fetal health outcomes across China and beyond. - Challenges and Opportunities in the IVD Industry
The in vitro diagnostics (IVD) industry faces numerous challenges, including regulatory complexities and the need for constant innovation. However, these challenges also present opportunities for companies like Zhejiang QL Biotech Co., Ltd to demonstrate leadership by delivering high-quality products like the iGFBP-1 Rapid Test Device. With a strong focus on research and development, the IVD industry in China is well-positioned to address global healthcare needs, providing solutions that enhance patient care and diagnostic accuracy.
Image Description