Detection of Canine C Reactive Protein: SARS-COV-2/Influenza A+B Antigen Combo Rapid Test Device by QL Biotech
PRODUCT SPECIFICATION
|
Brand |
QL |
Certificate |
CE |
|
Specimen |
Nasopharyngeal swabs/ Nasal swab |
Pack |
20T |
|
Reading Time |
10 minutes |
Contents |
Cassette , Buffer ,Package insert |
|
Storage |
2-30℃ |
Shelf Life |
2 years |
INTERPRETATION OF RESULTS
Same test procedure As SARS-COV-2 Antigen Rapid Test Device (Swab), The result Should be read at 10 minutes, Do not interpret the result after 20 minutes.
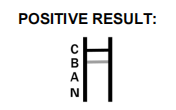
Possible Interpretation of Test Results:Flu B Positive:* A colored band appears in the control band region (C) and another colored band appears in the B region.

Flu A Positive:* A colored band appears in the control band region (C) and anothere colored band appears in the A region.

Flu A+B Positive:* A colored band appears in the control band region (C) and two other colored bands appear in the A region and B regions, respectively.
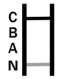
COVID-19 Positive:* A colored band appears in the control band region (C) and another colored band appears in the N region.

NEGATIVE RESULT:Only one colored band appears, in the control band region (C). No band appears in either test band region (A/B/N).
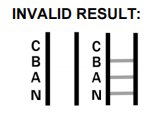
INVALID RESULT: Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor.
PERFORMANCE CHARACTERISTICS
Table: Flu A+B Rapid Test vs. other commercial brand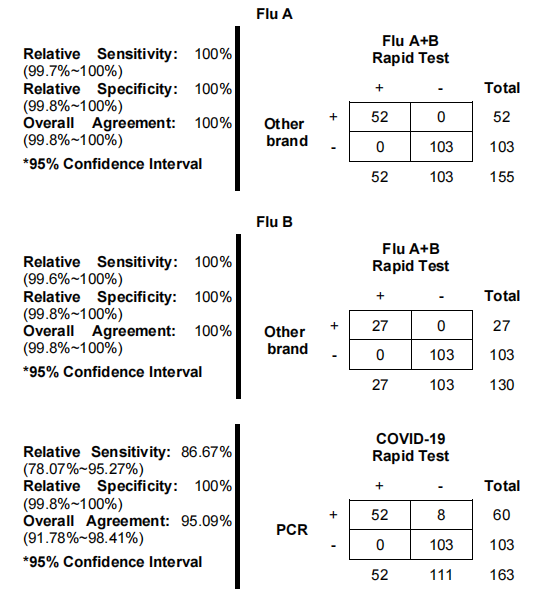
In using this device, please note that the same test procedure applies as with the SARS-COV-2 Antigen Rapid Test Device (Swab). Results should strictly be read within 10 minutes, and any interpretation after 20 minutes is deemed invalid. In conclusion, the SARS-COV-2/Influenza A+B Antigen Combo Rapid Test Device by QL Biotech is a reliable solution for the Detection of Canine C Reactive Protein. Its speed, accuracy, and convenience ensure that it is an ideal choice for every medical professional.


